Nutritional Aspects Related with Casein and Gluten Consumption in Autism Spectrum Disorder ASD
Nadia Isaac da Silva and Ivo Lebrun*
Laboratory of Biochemistry and Biophysics, Instituto Butantan, São Paulo, Brazil
- *Corresponding Author:
- Ivo Lebrun
Laboratory of Biochemistry and Biophysics,
Instituto Butantan, São Paulo,
Brazil,
E-mail: ivo.lebrun@butantan.gov.br
Received date: April 24, 2023, Manuscript No. IPJCND-23-16495; Editor assigned date: April 26, 2023, PreQC No. IPJCND-23-16495 (PQ); Reviewed date: May 10, 2023, QC No. IPJCND-23-16495; Revised date: May 17, 2023, Manuscript No. IPJCND-23-16495 (R); Published date: May 25, 2023, DOI: 10.36648/2472-1921.9.5.188
Citation: da Silva NI, Lebrun I (2023) Nutritional Aspects Related with Casein and Gluten Consumption in Autism Spectrum Disorder ASD. J Clin Nutr Diet Vol.9 No.5:188.
Abstract
The etiology of Autism Spectrum Disorder (ASD) is unknown, involves a complex interaction between multiple and varied susceptible genes, epigenetic and environmental factors. The diagnosis is made through clinical evaluation. First-line therapies are psychosocial treatments and educational interventions. On average 30% of people and with ASD consumes some drug or supplement. The options and effectiveness of the treatments are limited and therefore several lines of alternative treatment have emerged. The use of dietary restriction to reduce behavioral symptoms is widespread, with the most popular being the "theory of excess opioid peptides", which postulates that the consumption of gluten and casein contributes to the worsening of symptoms and a diet free of these proteins improves the clinical picture. Despite many data presented in the literature, there are still doubts about the mechanisms involved as well as about dietary efficacy. For all of the above, the topical review will address the opioid system, opioid peptides derived from gluten and casein, changes in the digestive tract of individuals with ASD, history of the onset of the theory, physiological mechanisms involved, brief description of the scientific literature, on the subject and the perspectives as a therapeutic instrument.
Keywords
Autism spectrum disorder; Diet; Gluten; Casein; Opioid peptides
Abbreviations
ASD: Autism Spectrum Disorder; BBB: Blood- Brain Barrier; CNS: Central Nervous System; DPPIV: Dipeptidyl Peptidase-4 (DPPIV, CD26; EC 3.4. 14.5); ECs: Microvascular Endothelial Cells; EECs: Enteroendocrine Cells; ENS: Enteric Nervous System; GFCF diet: Gluten-Free and Casein-Free diet; GIT: Gastrointestinal Tract; GM: Gut Microbiota; HPA: Hypothalamic-Pituitary-Adrenal axis; IB: Intestinal Barrier; MGB: Microbiota-Gut-Brain axis.
Introduction
Autism Spectrum Disorder (ASD) is a developmental disorder characterized by a framework that includes areas of social interaction, communication and behavior and sensory sensitivity [1]. The intensity and symptoms vary from mild to very severe manifestations; the prognosis of children with ASD is varied. Children with early diagnosis after growth became not completely independent adults [2]. In addition to symptoms such as persistence in performing routines, impairments in communication and language, a number of other non-specific problems are often present, such as anxiety, phobias, temper tantrums and aggression. Other common symptoms also relate to hyper or hyperactivity to sensory stimuli such as light, pain, or sound and other severe clinical conditions coexist with ASD [3].
Currently, there is a consensus on the alarming increase in prevalence over the last two decades. In 2000, the CDC (Centers for Disease Control and Prevention) started monitoring the prevalence of ASD among eight-year-old children in the USA. Initial reports estimated the prevalence rate at 6.7/1000, but the most recent report published in 2020 suggests an index of 18.5/1000, an increase of more than 115%. The male sex is most affected, according to studies; the observed ratio is 4 males to 1 female [4-7].
The etiology of ASD is unknown and may involve a complex interaction between multiple and varied susceptibility genes, epigenetic factors and environmental components [8,9]. At present, there are no specific laboratory tests that can confirm the disorder, so the characterization of ASD is basically done by clinical observation associated with the application of specific diagnostic criteria [10,11].
First-line treatments for children with ASD generally include psychosocial treatments and educational interventions, aimed at maximizing language acquisition, improving social and communication skills and ending maladaptive behaviors [12,13]. Psychopharmacologic treatment of children and adults with ASD is common in clinical practice. Approximately one-third of individuals with ASD use medications such as stimulants, tricyclic antidepressants, antipsychotics and many others for the disorder or for psychiatric and behavioral problems [14].
Despite the significant high economic and social costs, treatment options to improve the symptoms associated with ASD are limited. This scenario allows the development of various alternatives and approaches to the treatment of ASD, one of the most famous and also discussed in terms of its effectiveness in helping the ASD disorder is the introduction of restrictive or special diets.
The Relationship between Autism and Food Consumption
The first formal description of gastrointestinal symptoms and eating problems in individuals with ASD is present and in some cases described in the historic article by Kanner [15]. In 1961, Asperger, observed autistic behavior in individuals with celiac disease [16]. O'Banion, et al., published the paper that established the relationship between food consumption and exacerbation of the clinical status of ASD. They observed the behavior of an eight-year-old boy with ASD after food intake and concluded that corn, mushroom, tomato, sugar, dairy products and wheat exacerbated behavioral symptoms [17]. Several theories have been proposed regarding the relationship between food consumption and the exacerbation of ASD symptoms. The most popular is the "excess opioid peptide theory”, which postulates that the consumption of gluten and casein contributes to the worsening of symptoms otherwise a diet free of these proteins leads to an improvement in the clinical condition [18,19]. This diet is heavily promoted at events for parents of individuals with ASD. Currently, several books have been published and several websites provide information on the Gluten-Free and Casein-Free diet (GFCF diet) [20].
History of Theory of Excessive Opioid Peptides
In 1978, Kalat, wrote an article on the similarity between ASD and opioid addiction [21]. In the same year, Panksepp and coworkers developed several experiments with young animals of different species. They observed that after administration of low doses of psychoactive drugs and opioid peptides, the animals exhibited behavior similar to that of children with ASD. In 1979, based on the results obtained in an article entitled “A neurochemical theory of autism”, Panksepp, postulated the theory that ASD is an emotional disorder resulting from opioid overload [22].
Later studies with humans reinforced the theory. Analysis revealed a high concentration of β-endorphin in the cerebrospinal fluid and plasma of individuals with ASD [23,24]. Leboyer, et al., identified a high concentration of the fragment of the C-terminal region of β-endorphin in the plasma of children with ASD [20-29]. Tordjaman, et al., found significantly higher values of endorphins and Adrenocorticotrophic Hormone (ACTH) in children with ASD [26].
In parallel with these studies, Dohan researched the dietary habits of the South Pacific Islands region and associated the consumption of a diet free of wheat, rye, barley and oats by this population with a lower incidence of schizophrenia and also a higher prevalence of less severe cases [27]. In subsequent studies, he reported an improvement in the clinical condition of schizophrenics submitted to a gluten and/or casein-free diet [28,29]. Some research groups concluded that Dohan's theory applied to ASD.
Pieces of a Puzzle
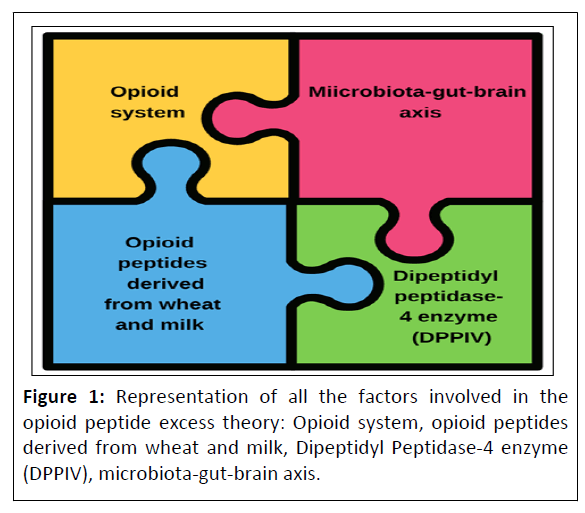
To understand the theory of opioid peptide excess it is important to know all the variables involved. Figure 1 illustrates the variables that will be presented in the following description of each aspect.
The opioid system
The opioid system is formed by a set of receptors and endogenous peptide ligands. The first receptors identified were μ (mu morphine), κ (kappa cetociclazocine) and δ (delta deferens ), which are divided into subtypes (eg., μ1 and μ2 receptors). As research progressed, others were identified as receptors ε (epsilon) and ORL-1. They are members of the family of receptors G protein coupled and their binding peptides opioid receptors are classified into two groups; typical and atypical opioid peptides [30].
Typical opioid peptides belong to three families of peptides derived from the precursors Proopiomelanocortin (POMC), Proenkephalin (PENK) or Prodynorphin (PDNY), which have been properly identified and cloned. They are expressed in the Central Nervous System (CNS) and various peripheral tissues and give rise to several active peptides including β-endorphin, met- and leu-enkephalin, dynorphin and neodynorphine respectively. This group presents in the N-terminal region a sequence the tetrapeptide Tyr-Gly-Gly-Phe, which confers the binding property with opioid receptors [31].
Atypical opioid peptides carry several amino acid sequences in the N-terminal region, generally conserving only the tyrosine amino acid at position 1. The human body produces atypical opioid peptides, for example: Orphanage/nociceptin, which is the endogenous ligand of the ORL-1 receptor derivative of the pro-nociceptin precursor. The exogenous opioid atypical peptides have also been identified in proteins from various sources, including food and skin of amphibians [32].
This system is crucial for the maintenance of homeostasis and survival of the organism and due to its wide distribution it regulates several physiological responses such as the transmission of nociception, cardiovascular activity and the circadian cycle. It also regulates levels of anxiety, depression, character, loco motor profile, memory, behavior and acts on the development of the CNS, as it operates on the proliferation, migration and differentiation of brain cells [30,31,33].
Opioid peptides derived from wheat and milk
One liter of milk contains approximately thirty-two grams of protein, consisting of two distinct fractions, casein (80% protein content) and skim protein (20% protein content). The largest groups of exogenous opioid peptides are the β -caseins released from the 60-70 sequence (Tyr-Pro-Phe-Pro-Gly-Pro-Ile-Pro-Asn- Ser-Leu) from β -casein. The most studied fragments are f60–63, f60–64, f60–65, f60–66 and f60-70, with the heptapeptide being the most potent of all peptides belonging to this family. β- casomorphines exhibit the μ-type opioid receptor ligand behavior [34-36].
Gluten corresponds to 80% of the total protein present in the grains; it is a mixture of more than one hundred polypeptides. It consists mainly of two reserve proteins, gliadins (prolamins) with a molecular weight ranging from 30 to 50 KDa and glutenins (glutelins) with a molecular weight between 69 to 88 KDa [37,38]. In 1987 Graf, et al., isolated a α-gliadin heptapeptide with affinity and for the μ type opioid receptor [39]. In their study, Fukudome and Yoshikawa identified and characterized four opioid peptides, namely exorfins derived from gluten A5, A4, B5, and B4. They exhibited δ-type opioid receptor ligand properties [34,40,41]. Gliadorfin-7, derived from α-gliadin, corresponds to the physiological action of casomorphins. The major common feature between gliadorfin-7 and β- casomorphin-7 is a tyrosine-proline at the N-terminus, followed by two additional proline amino acids in close sequence [36,42]. Table 1 shows the amino acid sequence of opiod peptides derived from casein and gluten.
| Source | Sequences | Peptide name | References |
|---|---|---|---|
| Bovine milk β-casein | Tyr-Pro-Phe-Pro | β-casomorphin-4 | [36] |
| Tyr-Pro-Phe-Pro-Gly | β-casomorphin-5 | ||
| Tyr-Pro-Phe-Pro-Gly-Pro | β-casomorphin-6 | ||
| Tyr-Pro-Phe-Pro-Gly-Pro-Ile | β-casomorphin-7 | ||
| Tyr-Pro-Val-Glu-Pro-Phe | neocasomorphin-6 | ||
| Gluten | Gly-Tyr-Tyr-Pro-Thr | gluten exorphins A5 | [40] |
| Gly-Tyr-Tyr-Pro | gluten exorphins A4 | ||
| Tyr-Gly-Gly-Trp-Leu | gluten exorphins B5 | ||
| Tyr-Gly-Gly-Trp | gluten exorphins B5 | ||
| Tyr-Pro-Ile-Ser-Leu | gluten exorphins C | [41] | |
| Tyr-Pro-Gln-Pro-Gln-Pro-Phe | gliadorphin -7 | [36] |
Table 1: Sequence of opioid peptides derived from gluten and casein.
Dipeptidyl Peptidase-4 enzyme (DPPIV)
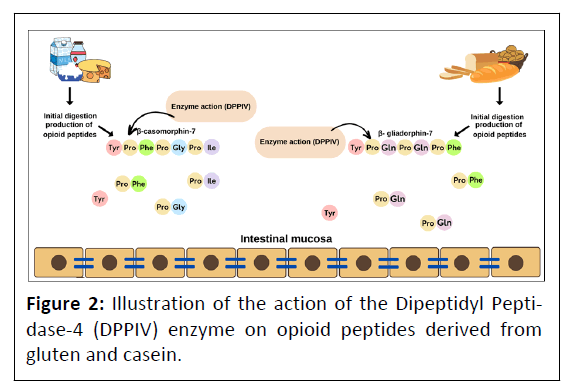
The enzyme Dipeptidyl Peptidase-4 (DPPIV, CD26; EC 3.4. 14.5) belongs to the family of serine proteases, is an amino peptidase with high specificity, crucial for the hydrolysis of small peptides having in the penultimate position of the N-terminal proline or alanine, generating di- and tripeptides to be transported through the intestinal wall. This enzyme is expressed in different cells in several mammalian species, it has been distributed in numerous organs/tissues such as liver, lung, intestinal epithelium, placenta, kidney, renal proximal tubules and neurons, as well as in body fluids such as urine, plasma and cerebrospinal fluid [43,44]. Exorphin-derived peptides from casein and gluten were identified as exogenous substrates for DPPIV and were inactivated under the influence of the enzyme. DPPIV deficiency and/or its lower enzyme activity have been proposed as possible causes of increased opioid levels in patients with ASD [45,46]. Figure 2 illustrates the processing action of the enzyme generating opioid peptides derived from casein and gluten.
Microbiota-gut-brain axis
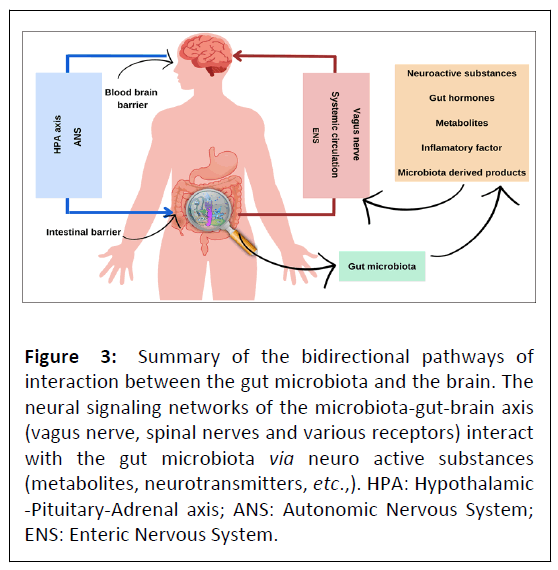
The knowledge of the existence of a continuous bidirectional communication between the gut and the brain was initially postulated by Hippocrates, Plato and Aristotle [47]. In recent decades, several studies have been developed with the aim of identifying and understanding the mechanisms involved in gutbrain communication and the regulatory role of the Gut Microbiota (GM) [48]. Currently, the concept of the Microbiota- Gut-Brain axis (MGB) encompasses the interaction of the hostmicrobiota and its communication with the CNS, endocrine chemical signaling, immune regulation, microbiota and metabolic effects, and barrier functions in the brain and gut [49,50]. Figure 3 summarizes the mechanisms involved in MGB.
Figure 3: Summary of the bidirectional pathways of interaction between the gut microbiota and the brain. The neural signaling networks of the microbiota-gut-brain axis (vagus nerve, spinal nerves and various receptors) interact with the gut microbiota via neuro active substances (metabolites, neurotransmitters, etc.,). HPA: Hypothalamic -Pituitary-Adrenal axis; ANS: Autonomic Nervous System; ENS: Enteric Nervous System.
The coordination of these factors plays an important role in maintaining a person's health. If there is an imbalance in the MGB, it can trigger symptoms (feeling of nausea, discomfort and/or pain), digestive tract diseases (irritable bowel syndrome, etc.), ASD, Parkinson's disease, epilepsy and migraine [50,51].
Routes of communication
Gut microbiota: The GM is a community of commensal and symbiotic microorganisms that reaches a density of more than 1012 cells/g content in the human colon [52]. Recent research has determined the total weight of intestinal microbes to be 1-2 kg [53]. In healthy adult individuals, 90% of the intestinal bacteria belong to 2 phyla: Bacteroidetes and Firmicutes. Proteobacteria, Actinobacteria, Fusobacteria and Verrucomicrobia make up the remaining 10% together with some species from the Archaea domain. The human GM also includes yeasts, bacteriophages and protists [54]. GM plays a key role in neurodevelopment. The onset of brain formation coincides with the coordinated development of the nervous system. The microbiome and its metabolic by-products play an active role in regulating early brain development. Disruption of this early developmental process can trigger a range of neurodevelopmental and neuropsychiatric disorders [55].
Communication between the GM and the CNS occurs via the microbial-metabolite pathway (short-chain fatty acids, secondary bile acids and tryptophan metabolites) and also via microbial production of neurotransmitters (e.g., Candida, Escherichia, Enterococcus and Streptococcus serotonin, Bifidobacterium and Lactobacillus GABA, Lactobacillus acetylcholine) [51,56]. Some intermediates, such as short-chain fatty acids, are able to cross the Intestinal Barrier (IB) to enter the systemic circulation and cross the Blood-Brain Barrier (BBB) [52,57]. The BBB does not allow neurotransmitters produced in the gut to reach the brain, except for GABA, which has transporters in the BBB. However, gut-derived neurotransmitters can indirectly influence the brain by acting in the Enteric Nervous System (ENS) [51,58]. The GM also has an intimate relationship with Enteroendocrine Cells (EECs), microorganisms directly or indirectly shape the functions of these cells, the diversity and composition of the microbiota influence the release of gut hormones such as Cholecystokinin (CKK), Peptide YY (PYY), Glucagon-Like Peptide-1 (GLP-1) and gastric inhibitory polypeptide [59].
Neural networks: The neural signaling networks of the MGB axis are responsible for important and rapid responses; this network is composed of the ENS, vagus nerve, spinal nerves and various receptors [49]. The ENS is characterized by an independently functioning neural system that innervates the entire Gastrointestinal Tract (GIT) and contains two bundles of ganglia: One in the sub mucosal plexus and the other in the myenteric plexus [60]. Its positioning allows it to respond directly or indirectly to the microbiota and their metabolites. It is largely responsible for the coordination of intestinal functions (motility and control of fluid movement) and acts in glucose homeostasis [61].
The vagus nerve is the tenth cranial nerve that extends from the brain to the abdomen. It regulates metabolic homeostasis, controls heart rate, motility and gastrointestinal secretions, plays a critical role in the regulation of the immune system, regulates pancreatic endocrine secretion and other visceral functions [62]. It can detect microbial signals in the form of bacterial metabolites or be influenced by microbiota-mediated modulation of ECCs and enterochromaffins in the intestinal epithelium [47,63]. The opioid system is an elaborate structure present in the GIT and the myenteric plexus of the ENS [64,65]. The distribution of opioid receptors in the GIT is heterogeneous; for example, in human and animal studies, opioid receptors of the μ, κ and δ subtypes were found to be localized in the GIT, but their relative distribution varies with GIT layer and region, as well as species [66].
The opioid system is involved in the modulation of intestinal motility and the secretion of various endogenous substances in the GIT [67]. The regulatory action of the opioid system on the GIT is also related to the fact that opioid peptides are expressed by different classes of enteric neurons, mainly in myenteric neurons projecting to the sphincter and in neurons of the descending enteric tracts [66].
Immune system: The immune system is responsible for maintaining a balance between homeostatic tolerance of commensal organisms and protection of the body against pathogenic microbial invasion. This system also plays an important role in mediating communication between the GM, the ENS and the brain [47,49,63]. Activation of the immune system is induced by the GM and occurs not only in the intestinal immune system, but also through long-distance regulation of immune cells in the brain [49]. The GM is involved in the regulation of innate and adaptive immunity, acting locally in the GIT and throughout the body [68].
Neuroendocrine system: The endocrine-neurocrine interaction between the hypothalamus, pituitary and adrenal gland in response to stress is known as the Hypothalamic- Pituitary-Adrenal axis (HPA) [55]. It is the main neuroendocrine system that participates in the regulation of various bodily processes in response to stressors (psychological, physical, infectious) and ensures an appropriate response to the stressor [69]. There is scientific evidence of an interaction between the HPA and the GM. Chronic HPA activation can affect the composition of the GM and increase gastrointestinal permeability, triggering gut dysbiosis [69].
Protection barriers: The GIT and CNS develop from closely related parts of the embryo, giving them common features that are specific and specialized vascular barriers called the BBB and the IB [51].
Blood-brain barrier: The BBB is a multicellular vascular structure that separates the CNS from the peripheral circulation. The central anatomical element of the BBB is the cerebral blood vessel, which is formed by microvascular Endothelial Cells (ECs) [70]. The BBB is present in the brain and spinal cord of most mammals and other organisms with a well-developed CNS. It is considered the major interface for blood-brain exchange [71,72]. ECs of the BBB have distinct morphological, structural and functional properties that distinguish them from other vascular endothelia: They express tight junctions, form a continuous cell lining without fenestrations and exhibit low levels of transcytosis. These characteristics limit the paracellular and transcellular movement of molecules through the cell layer [72,73]. The passage of molecules through the BBB is mediated by specific transporters (efflux transporters and solutes) that allow the delivery of nutrients to the brain and the extrusion of harmful compounds (e.g., toxins and xenobiotics). ECs exhibit low expression of leukocyte adhesion molecules, thereby preventing immune cell infiltration into the healthy CNS [70,71,74].
Intestinal barrier: The IB is a dynamic entity that interacts and responds to various internal and external stimuli, covering a surface area of approximately 400 m2 and requiring approximately 40% of the body's energy expenditure [75]. It is composed of physical elements (mucus, epithelial layer), humoral elements (defensins, IgA), immunological elements (lymphocytes, innate immune cells) and muscular and neurological elements [76]. The IB acts as a semipermeable barrier that allows nutrient uptake and immune sensing while limiting the transport of potentially harmful antigens and microorganisms [76,77]. The maintenance of intestinal functional barrier homeostasis is a process co-regulated by mediators: Epithelial cells, immune system cells, microbiota and the ENS. There are several lines of evidence supporting an association between abnormal gut permeability and gastrointestinal disorders [75,77,78]. In the population of people with ASD, the prevalence of gastrointestinal symptoms and diseases is high [79].
Evidences of changes in the microbiota-gut-brain axis in ASD
The first evidence of the relationship between gastrointestinal dysfunction and ASD was described in 1971 by Goodwin, Cowen and Goodwin, who observed intestinal malabsorption in a group of children with ASD [80]. The research findings stimulated the study of GIT in this population and several alterations were identified, such as: Low activity of digestive enzymes responsible for hydrolyzing carbohydrates and proteins, gastroesophageal reflux, food allergy and dysfunction of secretin production [79,81,82].
Several studies have reported a high incidence of inflammatory diseases in children with ASD and gastrointestinal symptoms who have undergone endoscopic and histologic examinations. The lesions identified are characterized by ileocolonic lymphoid nodular hyperplasia and ileocolitis. Some papers also report a high incidence of esophagitis, atypical focal gastritis and enteritis [83].
Studies of inflammatory lesions in the small intestine and colon of individuals with ASD compared to lesions in control groups have shown that the lesions seen in individuals with ASD differ from those seen in known inflammatory diseases (ulcerative colitis, gastritis, crohn's disease) [84-86]. Colonic lesions are characterized by a high intraepithelial density of T CD8+ cells and γδ T cells, a prominent infiltration of neutrophils and eosinophils, reduced expression of Human Leukocyte Antigen (HLA-DR) on the surface of colonic mucosal cells, suggesting a dominant T-helper 2 (Th2) immune response [85]. Research who analyzed the transcriptomic profile of gastrointestinal mucosal biopsy tissue and peripheral blood from individuals with ASD who manifest the above inflammatory condition reported a specific molecular profile [87]. Alessandria, et al., in a recent study, identified a high prevalence of duodenal intraepithelial lymphocytic infiltration in children with ASD and gastrointestinal symptoms, associated with an autoimmune response mechanism to gluten consumption specific to individuals with ASD [88].
The presence of dysbiosis in the gut of individuals with ASD is a problem that has been reported in several studies. Studies have described high growth of different species of Clostridium and Candida as well as members of the genus Sutterella [89-91].
Rose, et al., identified a biological signature in terms of immune dysfunction and specific microbiota composition in children with ASD who experience gastrointestinal symptoms. They found that this group of children with gastrointestinal symptoms had altered GM composition and elevated levels of mucosal-related cytokines. The authors also report the identification of an over-representation of the HP2 gene encoding zonulin, which increases the risk of increased intestinal permeability. They found that the fecal microbiota of children with ASD showed a significant decrease in the Bacteroidetes/ Firmicutes ratio and a trend toward an increased incidence of Desulfovibrio spp in the microbiota [92]. The nature of the changes identified in the microbiota of children with ASD is not yet known. One of the probable causes is the frequent occurrence of infectious diseases in this population during childhood, which leads to the constant use of antibiotics, resulting in an imbalance of the GM.
In recent years, there has been increasing evidence that the high prevalence of GIT disorders in children with ASD is related to genetic factors. Bernier, et al., performed re-sequencing of the CHD8 gene in 3.730 children with developmental delay or ASD. Fifteen mutations were identified in the study population. The authors concluded that mutations in the CHD8 gene are associated with an increased likelihood of an ASD diagnosis, which is associated with some features such as macrocephaly and gastrointestinal complaints [93]. Campbel, et al., identified the variant rs1858830 in the MET gene, which reflects a single nucleotide polymorphism associated with the phenotype of children with ASD affected by GIT comorbidities [94].
Another gene that may be associated with increased susceptibility to ASD and GIT dysfunction is SLC6A4, which encodes for serotonin membrane transport [95,96]. The polymorphism identified in individuals with ASD is associated with dysfunction of serotonin metabolism by the GIT. Alterations in serotonin levels and serotonergic signaling in the GIT are associated with a variety of pathological conditions, including irritable bowel syndrome, inflammatory bowel syndrome and idiopathic constipation [97,98].
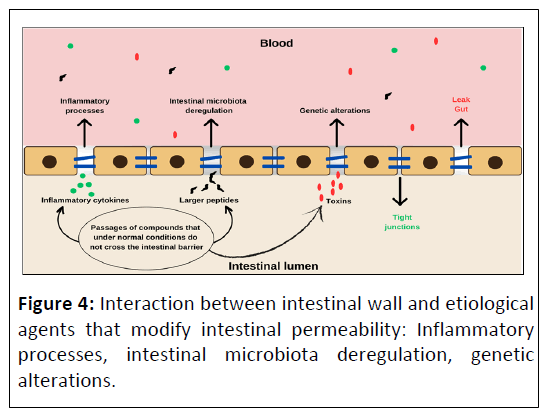
Altered intestinal permeability may also be an intrinsic feature of ASD. In the study by D'Eufemia, et al., the occurrence of intestinal mucosal damage was determined using the intestinal permeability test (lactulose/mannitol test) in 21 autistic children who did not have clinical and laboratory findings consistent with known intestinal disorders. An altered intestinal permeability was found in 43% autistic patients, but in none of the 40 volunteers in the control group [99]. de Magistris and colleagues (2010) investigated in patients with autism as well as in their first-degree relatives the hypothesis of high prevalence of permeable bowel by means of the lactulose/mannitol test. The results pointed to a high percentage of abnormal gut permeability values in autistic patients (36.7%) and their relatives (21.2%) compared to neuro typical individuals (4.8%). The authors concluded that altered intestinal permeability may be an intrinsic characteristic for a subgroup of people with ASD and that the altered intestinal permeability found in first-degree relatives suggests the presence of a hereditary factor [100]. Figure 4 illustrates the IB with altered permeability and its possible agents.
There are mechanisms that assist in the regulation of BBB permeability. Many mechanisms known today present in an altered form in individuals with ASD.
Animal studies have demonstrated the relationship of BBB permeability to the GM via gut-derived neurotransmitters and bacterial metabolites [101]. Rodent models report a loss of normal GM results in increased BBB permeability, while a pathogen-free gut microbiota restores BBB functionality [102].
Systemic or localized inflammatory processes in the CNS can alter the permeability of the BBB by means of some mechanisms already described in literature such as altered signaling, increased cellular traffic, increased solute permeability and finally direct damage [103]. Several studies in postmortem autistic brains have reported immunological changes that characterize different pictures of neuroinflammation. Findings include prominent activation of the microglia, increased inflammatory cytokines and production of chemokines including, Interferon Gamma (IFN-γ), Interleukin-1β (IL-1β), Interleukin-6 (IL-6), Interleukin-12p40 (IL-12p40), Tumor Necrosis Factor-alpha (TNF-α). High Concentration of Chemokine C-C motif Ligand (CCL)-2 has been identified in brain tissues and also in cerebrospinal fluid [104-106].
Gene expression profiling of postmortem brain tissues from individuals with ASD revealed increased messenger RNA transcript levels of several immune system-related genes, particularly those associated with neuroinflammation processes [107]. Voineagu, et al., observed the transcription pattern of neuronal cells and concluded that the gene co-expression network reflects abnormalities of cortical modulation in the brains of individuals with ASD. These findings were associated with alterations in microglia and immune activation; suggesting a causal role for immune dysregulation in the course of neurological dysfunction and synapse plasticity of the brain of individuals with ASD [108]. Fiorentino, et al., identified in the brains of individuals with ASD altered expression of genes associated with BBB integrity, increased neuroinflammation and intestinal barrier integrity [109].
Altered BBB permeability in autistics may be an inherent condition for a subgroup of people with ASD. Novarino, et al., idetified inactivating mutations in the Branched Chain Ketoacid Dehydrogenase Kinase (BCKDK) gene in inbred families with autism. This genotype corresponds to abnormalities in the Branched-Chain Amino Acid (BCAA) catabolic pathway in the BBB [110]. Jagadapillai, et al., identified genetic alterations in autistics related to the expression of neurovascular signaling. Modification of CNS vascularization may alter the permeability of the BBB [111].
Intense and chronic exposure of opioid receptors to their endogenous or exogenous ligands (opioid peptides of dietary or synthetic origin) can trigger symptoms and itemize the clinical picture of some diseases [112,113]. Studies in animal and human models with different methodological designs have described that chronic opioid use can lead to dysregulated immune response, increased intestinal barrier permeability, bacterial translocation, increased risk of intestinal sepsis, as well as symptoms of abdominal distension [114].
Systemic opioids can alter intestinal motility. During periods of opioid administration, gut motility may decrease, whereas during periods of withdrawal, gut motility may increase [115,116]. Lee and colleagues identified changes in the GM of rodents subjected to intermittent and continuous morphine administration [117].
Opioid receptors are present on immune system cells such as B cells, T cells and macrophages; therefore, chronic opioid use may alter immune system function [114]. In a study using a nonhuman primate animal model, it was observed that morphine has the ability to produce immunosuppressive effects, attenuate T-cell maturation, alter cytokine secretion and decrease the production of proteins that mediate energy metabolism, signaling, and maintenance of cell structure [118].
In the study by Meng, et al., changes in the immune system after morphine administration were observed to lead to intestinal membrane disruption through a mechanism of action that degrades the organization of tight junctions. The change in intestinal wall structure occurred through modulation of Toll-like receptor-dependent Myosin Light Chain Kinase (MLCK) [119]. Banerjee, et al., observed in a rodent study that morphineinduced disruption of the GM triggers an inflammatory response initiated by IL-17 and sustained by IL-6 [120].
The theory of excessive opioid peptides
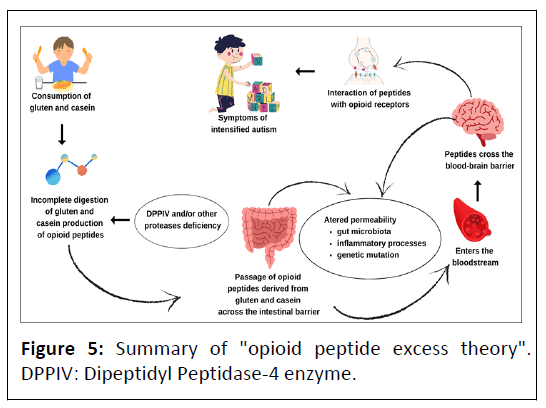
The theory of opioid system overload by exogenous foodderived peptides in individuals with ASD or schizophrenia postulates that deficiency of the enzyme DPPIV and/or other proteases that cleave the amino acid proline bond leads to excessive production of opioid peptides derived from gluten and casein in the intestinal lumen. Related to this fact, the increased permeability of the intestinal wall due to various conditions and diseases allows the passage of opioid peptides into the bloodstream. Thus, these compounds, because of their affinity for opioid receptors and ability to cross the blood-brain barrier, trigger an overload of the opioid system, resulting in dysfunction in the brain and also in peripheral systems, contributing to the worsening of symptoms of ASD [18,34,89,121]. Figure 5 illustrates the summary of the opioids peptides excess theory.
This theory gained strength with the publication of studies that analyzed the profile of protein compounds in body fluids using a gel filtration method, High Performance Liquid Chromatography (HPLC) and high performance Liquid Chromatography coupled with Mass Spectrometry (LC-MS). The results showed that high concentrations of peptides and the presence of exorphins derived from gluten and casein have been described in the urine of individuals with ASD [122-125]. Shanahan, et al., using Electrospray Ionization Mass Spectrometry (ESI-MS) identified β-casomorphin fragments, α- gliadin and other compounds of high biological potential that are not of dietary origin in the urine of children with ASD [126].
Sokolov, et al., developed an ultrasensitive Enzyme-Linked Immune Sorbent Assay (ELISA) method for the detection and quantification of casein-derived opioid peptides in urine. In this work, the authors identified a high concentration of β- casomorphine-7 in urine samples from individuals with ASD compared to control groups and also observed a correlation between the concentration of β-casomorphine-7 and the severity of the clinical picture [127]. In a recent study, Parashar, et al., developed a non-SELEX method for aptamer selection against β-casomorphin-7 peptide. According to the authors, this method allows the detection of very low levels of β- casomorphin-7 in urine samples in a practical and safe manner [128].
Scientific evidence on Gluten and Casein-Free diet (GFCF diet)
Some studies have reported an association between the GFCF diet and progress in social behavior, cognitive development and communication in individuals with ASD. Reichelt, Ekrem and Scott, in one of the first studies to evaluate the efficacy of the GFCF diet, followed the clinical course of fifteen children between the ages of three and seventeen diagnosed with ASD during a dietary intervention. In this work, three diets were tested: Free of gluten and reduced in casein, free of casein and reduced in gluten, or free of gluten and casein. The subjects were assigned to one of the three interventions and the criterion used to determine the diet was the urinary peptide profile determined by gel filtration technique. The children were followed for one year and 60% received the medication thioridazine from the second to the third month. Behavior was assessed using a questionnaire administered to parents and teachers of individuals with ASD. The results indicated behavioral improvement in 86% of the participants [129].
A study conducted in Norway used the same methodology, but without the use of pharmacological treatment. The research showed that the children with ASD who completed the study (80%) showed improvement in their clinical picture, including attenuation of bizarre behavior and social isolation, as well as reduced levels of peptides in their urine. This research lasted four years and included fifteen individuals diagnosed with ASD between the ages of 6 and 22 who had "abnormal" urinary peptide patterns [130,131]. In the research developed by Lucarelli, et al., performed with thirty-six children with ASD it was observed the improvement in their performance, after eight weeks of adherence to a diet free from cow's milk [132]. Adams and Conn reported an improvement in the behavior of individuals with ASD on the FBCF diet associated with the use of a nutritional supplement consisting of vitamin B6, magnesium and other nutrients not disclosed by the researchers [133].
Studies with more controlled methodology were published later. In the study by Knivsberg, et al., twenty children with ASD and abnormal levels of opioid peptides in urine were observed. The participants were divided into two groups, with ten autistic children on a gluten and case in free diet and the other group as a control for a period of one year. The results showed a significant improvement in symptoms in the group of children with ASD who consumed the GFCF diet compared to the control group [134].
Whiteley, et al., evaluated the efficacy of a casein and glutenfree diet. Seventy-two children with ASD between the ages of four and ten years participated in this study. The concentration of peptides and/or the presence of trans-Indolyl-3- Acryloylglycine (IAcrGly) were used as an inclusion criterion. The participants were divided into two groups: 38 children with autism consumed a GFCF diet and 34 children consumed a control diet for a period of two years. The results showed a significant improvement in attention deficit and hyperactivity in the autistic children who consumed the GFCF diet compared to the control group [135]. Ghalichi, et al., in a randomized trial, divided eighty children diagnosed with ASD into two groups. One group received a gluten-free diet for six weeks, while the other control group received a regular diet (n=40). Of the eighty children studied, 53.9% had gastrointestinal changes. In the gluten-free diet group, there was a significant decrease in the prevalence of gastrointestinal symptoms and a significant decrease in behavioral disturbances. In this study, the parameters were measured using questionnaires [136].
In a recent study, Adams, et al., developed a 12 month randomized controlled trial of an open nutritional intervention. Participants were sixty seven individuals with ASD, aged 3-58 years and fifty neuro typical controls of similar age and gender. Treatment began with a specific vitamin/mineral supplement and additional treatments were added sequentially, including essential fatty acids, Epsom salt, carnitine, dietary enzymes and a GFCF diet. The researchers observed a significant improvement in ASD symptoms in individuals in the treated group compared to the control group [137].
Some case studies also report improvement in the clinical condition of individuals with ASD following adherence to dietary intervention with gluten and casein restriction [138,139]. Crosssectional studies have administered questionnaires to parents and caregivers of individuals with ASD to assess perceptions of change in clinical status after intervention and also in reported symptoms improvement [140,141].
Some well controlled studies have failed to replicate the positive results. Elder, et al., tested the efficacy of a gluten and casein free diet in the treatment of ASD through a randomized, crossover, double-blind clinical trial in which the sample consisted of fifteen children aged 2 to 16 years with ASD. Data on behavioral symptoms and urinary peptide levels were collected during the twelve week dietary intervention. The data were not statistically significant, although several parents reported improvements in their children [142].
Seung, et al., in a double blind randomized clinical trial in which thirteen children aged 2 to 16 years with a diagnosis of ASD were placed on a gluten free and GFCF diet for six weeks, analyzed verbal and nonverbal communication using videos recorded at home while playing with their parents. The Friedman test was used to assess verbal responses, imitation of sounds and lexical production. The results showed that there was no statistical evidence of symptom improvement [143].
In a prospective randomized controlled trial, Johnson, et al., examined the effectiveness of a GFCF diet intervention as well as a healthy, low-sugar diet. Twenty-two children aged 3 to 5 years participated in this study. The research lasted three months and although researchers and parents reported improvements in behavioral symptoms characteristic of ASD, no statistically significant difference was observed between treatment groups [144].
The double-blind, placebo-controlled clinical trial by Hyman, et al., observed the efficacy of the GFCF diet over a thirty-week period. Fourteen children aged 3 to 5 years participated in the study. The evaluation of the effectiveness was done by observing the characteristics of feces and behavior. The result showed that the diet did not promote statistically significant effects on measures of physiological functioning, behavioral problems or symptoms of ASD [145].
Gonzalez-Domenech, et al., developed crossover clinical trial studies. The goal of both studies was to determine the influence of a GFCF diet on the behavior of children and adolescents diagnosed with ASD and to explore the possible association between ASD symptoms and urinary concentrations of β- casomorphine. In both studies, patients followed a normal diet (including gluten and casein) for a specified period of time and then underwent a switch to a gluten and casein free diet. The order of intervention (normal diet or GFCF diet) was randomized. The participants' progress was monitored by means of questionnaires and the concentration of beta-casomorphine in urine samples using chromatographic techniques. The results of the research reported that the consumption of a GFCF diet did not provide patients with significant changes in behavioral symptoms nor in urinary beta-casomorphine concentrations using chromatographic detection [146,147].
Final Considerations
The evidence reported in studies provides some support for the opioid overload theory, but more research is needed to fully understand and unequivocally confirm the hypothesis. Although some studies are well controlled and show positive results, the efficacy of the GFCF diet is still questioned because most of the published research had methodological flaws such as lack of a control group, administration of medications and supplements in conjunction with the dietary intervention, Diversity of diagnoses of participants (individuals with ASD and Rett syndrome), limited number of participants, wide age range of participants, male and female participants in the same group, heterogeneous nutritional interventions and the effectiveness of the diet in most studies was assessed by psychological tests and behavioral observations directed at teachers, therapists and relatives of individuals with ASD [148,149].
The heterogeneity of research results related to the mechanisms involved, such as changes in the GIT, makes it essential to develop research with a larger sample size to effectively confirm the relationship between ASD and the various physiological changes identified, in addition to a better control of the individuals tested for greater homogeneity of the groups and above all, to define factors that may contribute more or less to the success of the diet therapeutic approach, including the immune status of individuals, the state of the digestive tract system, including digestive enzymes. Genomic research on ASD may play an important role because, as mentioned above, studies have identified the genetic aspects related to the microbiota-gut-brain connection and the expression of enzymes and other key compounds in the process [18,150].
Another relevant aspect that reduces the reliability of the results obtained in several studies stems from the fact that the behavioral analysis is subjective and can be influenced by the expectation of improvement of the clinical picture created by teachers and relatives. It is also important to point out that behavioral analysis does not allow distinguishing how far the progression of the disease is related to dietary or psychoeducational interventions and/or pharmacological they receive in clinics and specialized outpatient clinics [18,149,151]. In the study by Hyman, et al., a high placebo effect was observed in the control group. Outcomes were measured by questionnaires and scales of physiological function [152].
For the diagnosis of individuals with ASD who have a high concentration of circulating opioid peptides, as well as for the effective control of the progression of the GFCF diet, it is essential to develop safe and easily reproducible biological markers. The analysis of the urinary opioid peptide profile has been the most adopted and studied in the studies. Despite the efforts of several research laboratories, only a few have managed to develop methods that successfully identify and quantify the opioid peptides derived from gluten and casein in urine. The lack of safe and easily reproducible biomarkers has limited the number of human studies and therefore part of the knowledge about the effect of gluten- and casein-derived exorphins on the opioid system of individuals with ASD has been based on results from in vivo animal and in vitro experiments.
The GFCF diet may cause nutritional deficiencies and affect the nutritional status of children with autism. Studies have found that children with autism who consumed the GFCF diet may have weight and body mass index inappropriate for their age, impaired bone development, inadequate nutrient intake and low plasma levels of essential amino acids [153-155].
Evaluating the results of surveys developed to identify opioid peptides in the urine of individuals with ASD, as well as clinical investigations and genetic alterations in the GIT and immune system, one can accept the hypothesis that specific physiological conditions are present in subgroups of individuals with ASD [156]. Age is considered a critical factor for a positive response to the GFCF diet. According to Pedersen, et al., children aged 7-9 years show a better response to the intervention [157].
The duration of the research can also be a variant that can compromise the results. Studies that opted for a short duration of nutritional intervention obtained negative results. Research that opted for a long period obtained positive results [150]. Researchers indicate a minimum time of 3 months to observe positive results. This follow-up time is established based on the remaining activity of gluten and its bio products in patients with celiac disease after elimination of this protein from the diet [1]. However, research groups with positive results suggest an even longer intervention period of more than 6 months [150].
The protein composition of cow's milk is influenced by the genetic variation of the producing animal and for this reason some regions may not provide milk and its derivatives that contain precursor proteins of casomorphins with opioid activity that may have an effect on the CNS. The β-casein can present as one of two main genetic types: A1 and A2 [42]. Commercially produced milk in many countries contains predominantly A1 casein or a combination of A1 and A2. The distinctive structure between these 2 forms of β-casein is the presence of histidine in A1 and proline in A2 at position 67 of this protein [158].
In β-casein A1 and β-casein A1&A2, the release of β- casomorphin 7 is facilitated by the presence of a histidine at position 67 and the C-terminal bond between positions 66 and 67 is easily cleaved by carboxyl peptidases such as elastase. In contrast, in β-casein A2, the amino acid at position 67 is another proline, which leads to preferential cleavage of the longer β- casomorphin 9 peptide and severely limits the formation of β- casomorphin 7 during in vivo digestion. β-casomorphin 9 is an opioid peptide with antihypertensive and antioxidant properties [159,160].
Another issue that should be raised is that there is evidence that gluten and casein may interfere in the clinical picture of ASD through other mechanisms. In an in vitro study, the effect of morphine, β-gliadorphine, bovine β-casomorphine and human β-casomorphine on the cysteine uptake system in cultured human neuronal and gastrointestinal epithelial cells was observed through the activation of opioid receptors. The study reports that food and morphine-derived opioid peptides can modulate cysteine uptake with subsequent effects on cellular redox and methylation status, leading to global changes in DNA methylation and gene transcription [161].
Opioid peptides derived from casein and gluten may also trigger a systemic immune reaction in a subset of individuals with ASD. Lucarelli, et al., found high levels of antigen-specific antibodies IgA to casein, α-lactalbumin and β-lactoglobulin and IgG and IgM to casein in blood samples from autistic children [132]. In Jyonouchi's research it was observed that human Peripheral Blood Mononuclear Cells (PBMCs) obtained from children with ASD produced higher amounts of Tumor Necrosis Factor-a (TNF-a)/Interleukin-12 (IL-12) than those obtained from controls when challenged with casein, β-lactoglobulin and α- lactalbumin [162]. Vojdani and colleagues described the presence of high concentration of IgG, IgM or IgA immunoglobulin antibodies against gliadin in serum of individuals with ASD [163]. de Magistris, et al., in their research concluded that the immune system of a subgroup of people with ASD is triggered by gluten and casein through elevated production of antibodies against α-gliadin (anti α–gliadin IgG-IgA and anti α-gliadin deamidated peptides IgA and IgG) IgG to casein [164]. Corroborating with the laboratory studies Xu and colleagues in a cross-sectional study in which data from 199,520 children aged 3 to 17 years who participated in the U.S. National Health Interview Survey from 1997 to 2016 were used identified high prevalence of food allergy in autistic children [165].
Conclusion
Despite the best efforts of researchers, the opioid peptide excess theory needs a more solid scientific foundation. The flaws in study methodology, along with the lack of reliable biomarkers to identify individuals who may respond positively to nutritional therapy, make this hypothesis ineffective in understanding the relationship between nutrition and autistic behavior. For all of these reasons, the development of new research to address all of the issues surrounding this theory is essential. Randomized clinical trials with larger numbers of participants are needed to explore the long-term effects of this diet on the nutritional and psychological status of individuals with ASD. The results of the research move towards the understanding that the prescription of a GFCF diet is indicated for a subgroup of people with ASD who present symptoms of GIT alterations and food allergies or intolerances, therefore the effectiveness of this approach should be preceded by a case-by-case approach, because the complexity of ASD and its multiple determinants do not guarantee a positive evolution through a single procedure, which, by the data presented, does not invalidate its usefulness and remains an alternative approach to the condition of ASD, which is certainly a multifactorial disease with many components.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Author Contributions
Conceptualization: N.I.S, I.L, Visualization: N.I.S, I.L; Writingoriginal draft: N.I.S, I.L; Writing review and editing: N.I.S, I.L; Project administration: I.L.
Funding
This work was supported by Fundação Butantan.
References
- https://doi.org/10.1176 /appi.books.9780890425787
- Bertoglio K, Hendren RL (2009) New developments in autism. Psychiatr Clin North Am 32: 1-14.
[Crossref], [Google Scholar], [Indexed]
- Styles M, Alsharshani D, Samara M, Alsharshani M, Khattab A, et al. (2020) Risk factors, diagnosis, prognosis and treatment of autism. Front Biosci 25: 1682-1717.
[Crossref], [Google Scholar], [Indexed]
- Centers for Disease Control and Prevention (2007) Prevalence of autism spectrum disorders-autism and developmental disabilities monitoring network, six sites, United States, 2000. MMWR Surveill Summ 56: 1-11.
[Google Scholar], [Indexed]
- Maenner MJ, Shaw KA, Baio J, Washington A, Patrick M, et al. (2020) Prevalence of autism spectrum disorder among children aged 8 years autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveill Summ 69: 1-12.
[Crossref], [Google Scholar], [Indexed]
- Jacquemont S, Coe BP, Hersch M, Duyzend MH, Krumm N, et al. (2014) A higher mutational burden in females supports a "female protective model" in neurodevelopmental disorders. Am J Hum Genet 94: 415-25.
[Crossref], [Google Scholar], [Indexed]
- Salari N, Rasoulpoor S, Rasoulpoor S, Shohaimi S, Jafarpour S, et al. (2022) The global prevalence of autism spectrum disorder: A comprehensive systematic review and meta-analysis. Ital J Pediatr 48: 112.
[Crossref], [Google Scholar], [Indexed]
- Bölte S, Girdler S, Marschik PB (2019) The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol Life 76: 1275-1297.
[Crossref], [Google Scholar], [Indexed]
- Emberti GL, Mazzone L, Benvenuto A, Fasano A, Alcon AG, et al. (2019) Risk and protective environmental factors associated with autism spectrum disorder: Evidence-based principles and recommendations. J Clin Med 8: 217.
[Crossref], [Google Scholar], [Indexed]
- Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J (2018) Autism spectrum disorder. Lancet 392: 508-520.
[Crossref], [Google Scholar], [Indexed]
- Masi A, DeMayo MM, Glozier N, Guastella AJ (2017) An overview of autism spectrum disorder, heterogeneity and treatment options. Neurosci Bull 33: 183-193.
[Crossref], [Google Scholar], [Indexed]
- Freeth M, Sheppard E, Ramachandran R, Milne E (2013) A cross-cultural comparison of autistic traits in the UK, India and Malaysia. J Autism Dev Disord 43: 2569-83.
[Crossref], [Google Scholar], [Indexed]
- Jiang CC, Lin LS, Long S, Ke XY, Fukunaga K, et al. (2022) Signalling pathways in autism spectrum disorder: Mechanisms and therapeutic implications. Signal Transduct Target Ther 7: 229.
[Crossref], [Google Scholar], [Indexed]
- LeClerc S, Easley D (2015) Pharmacological therapies for autism spectrum disorder: A review. P T 40: 389-397.
[Google Scholar], [Indexed]
- Kanner L (1943) Autistic disturbances of affective contact. Nervous Child 2: 217-250.
[Google Scholar], [Indexed]
- Asperger H (1961) Psychopathology of children with coeliac disease. Ann Paediatr 197: 346-351.
[Google Scholar], [Indexed]
- O'Banion D, Armstrong B, Cummings RA, Stange J (1978) Disruptive behavior: A dietary approach. J Autism Child Schizophr 8: 325-37.
[Crossref], [Google Scholar], [Indexed]
- Baspinar B, Yardimci H (2020) Gluten-free casein-free diet for autism spectrum disorders: Can it be effective in solving behavioural and gastrointestinal problems? Eurasian J Med 52: 292-297.
[Crossref], [Google Scholar], [Indexed]
- Christison GW, Ivany K (2006) Elimination diets in autism spectrum disorders: Any wheat amidst the chaff? J Dev Behav Pediatr 27: S162-71.
[Crossref], [Google Scholar], [Indexed]
- Van-DeSande MM, VanBuul VJ, Brouns FJ (2014) Autism and nutrition: The role of the gut-brain axis. Nutr Res Rev 27: 199-214.
[Crossref], [Google Scholar], [Indexed]
- Kalat JW (1978) Speculations on similarities between autism and opiate addiction. J Autism Child Schizophr 8: 477-479.
[Crossref], [Google Scholar], [Indexed]
- Panksepp J (1979) A neurochemical theory of autism. Trends Neurosci 2: 174-77.
[Crossref], [Google Scholar]
- Gillberg C, Terenius L, Lönnerholm G (1985) Endorphin activity in childhood psychosis. Spinal fluid levels in 24 cases. Arch Gen Psychiatry 42: 780-783.
[Crossref], [Google Scholar], [Indexed]
- Ross DL, Klykylo WM, Hitzemann R (1987) Reduction of elevated CSF beta-endorphin by fenfluramine in infantile autism. Pediatr Neurol 3: 83-86.
[Crossref], [Google Scholar], [Indexed]
- Leboyer M, Bouvard MP, Recasens C, Philippe A, Guilloud-Bataille M, et al. (1994) Difference between plasma N- and C-terminally directed beta-endorphin immunoreactivity in infantile autism. Am J Psychiatry 151: 1797-801.
[Crossref], [Google Scholar], [Indexed]
- Tordjman S, Anderson GM, McBride PA, Hertzig ME, Snow ME, et al. (1997) Plasma beta-endorphin, adrenocorticotropin hormone, and cortisol in autism. J Child Psychol Psychiatry 38: 705-15.
[Crossref], [Google Scholar], [Indexed]
- Elder JH (2008) The gluten-free, casein-free diet in autism: An overview with clinical implications. Nutr Clin Pract 23: 583-588.
[Crossref], [Google Scholar], [Indexed]
- Dohan FC (1966) Cereals and schizophrenia data and hypothesis. Acta Psychiatr Scand 42: 125-52.
[Crossref], [Google Scholar], [Indexed]
- Dohan FC, Grasberger JC (1973) Relapsed schizophrenics: Earlier discharge from the hospital after cereal-free, milk-free diet. Am J Psychiatry 130: 685-688.
[Crossref], [Google Scholar], [Indexed]
- Fricker LD, Margolis EB, Gomes I, Devi LA (2020) Five decades of research on opioid peptides: Current knowledge and unanswered questions. Mol Pharmacol 98: 96-108.
[Crossref], [Google Scholar], [Indexed]
- Palmer CB, Meyrath M, Canals M, Kostenis E, Chevigné A, et al. (2022) Atypical opioid receptors: Unconventional biology and therapeutic opportunities. Pharmacol Ther 233: 108014.
[Crossref], [Google Scholar], [Indexed]
- Janecka A, Fichna J, Janecki T (2004) Opioid receptors and their ligands. Curr Top Med Chem 4: 1-17.
[Crossref], [Google Scholar], [Indexed]
- Koneru A, Satyanarayana S, Rizwan S (2009) Endogenous opioids: Their physiological role and receptors. Global J Pharmacol 3: 149-53.
- Liu Z, Udenigwe CC (2019) Role of food-derived opioid peptides in the central nervous and gastrointestinal systems. J Food Biochem 43: e12629.
[Crossref], [Google Scholar], [Indexed]
- Meisel H (1998) Overview on milk protein-derived peptides. Inter Dairy J 8: 363-373.
[Crossref], [Google Scholar]
- Tyagi A, Daliri EB, Kwami OF, Yeon SJ, Oh DH (2020) Food-derived opioid peptides in human health: A review. Int J Mol Sci 21: 8825.
[Crossref], [Google Scholar], [Indexed]
- https://agris.fao.org/agris-search/search.do?recordID=GB9011335
- https://agris.fao.org/agris-search/search.do?recordID=US9123073
- Graf L, Horvath K, Walcz E, Berzetei I, Burnier J (1987) Effect of two synthetic alpha-gliadin peptides on lymphocytes in celiac disease: Identification of a novel class of opioid receptors. Neuropeptides 9: 113-122.
[Crossref], [Google Scholar], [Indexed]
- Fukudome S, Yoshikawa M (1992) Opioid peptides derived from wheat gluten: Their isolation and characterization. FEBS Lett 296: 107-111.
[Crossref], [Google Scholar], [Indexed]
- Fukudome S, Yoshikawa M (1993) Gluten exorphin C: A novel opioid peptide derived from wheat gluten. FEBS Lett 316: 17-9.
[Crossref], [Google Scholar], [Indexed]
- Woodford KB (2021) Casomorphins and gliadorphins have diverse systemic effects spanning gut, brain and internal organs. Int J Environ Res Public Health 18: 7911.
[Crossref], [Google Scholar], [Indexed]
- Al-Badri G, Leggio GM, Musumeci G, Marzagalli R, Drago F, et al. (2018) Tackling dipeptidyl peptidase IV in neurological disorders. Neural Regen 13: 26-34.
[Crossref], [Google Scholar], [Indexed]
- Zhong J, Gong Q, Goud A, Srinivasamaharaj S, Rajagopalan S (2015) Recent advances in dipeptidyl-peptidase-4 inhibition therapy: Lessons from the bench and clinical trials. J Diabetes Res: 606031.
[Crossref], [Google Scholar], [Indexed]
- Cieà ?lià ?ska A, Kostyra E, Savelkoul HFJ (2017) Treating autism spectrum disorder with gluten free and casein-free diet: The underlying microbiota-gut-brain axis mechanisms. HSOA Journal of Clinical Immunology and Immunotherapy.
- JarmoÃ
?owska B, BukaÃ
?o M, Fiedorowicz E, CieÃ
?liÃ
?ska A, Kordulewska NK, et al. (2019) Role of milk-derived opioid peptides and proline dipeptidyl peptidase-4 in autism spectrum disorders. Nutrients 11: 87.
[Crossref], [Google Scholar], [Indexed]
- Margolis KG, Cryan JF, Mayer EA (2021) The microbiota-gut-brain axis: From motility to mood. Gastroenterology 160: 1486-1501.
[Crossref], [Google Scholar], [Indexed]
- Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, et al. (2019) The microbiota-gut-brain axis. Physiol Rev 99: 1877-2013.
[Crossref], [Google Scholar], [Indexed]
- Chang L, Wei Y, Hashimoto K (2022) Brain-gut-microbiota axis in depression: A historical overview and future directions. Brain Res Bull 182: 44-56.
[Crossref], [Google Scholar], [Indexed]
- Holzer P, Farzi A (2014) Neuropeptides and the microbiota-gut-brain axis. Adv Exp Med Biol 817: 195-219.
[Crossref], [Google Scholar], [Indexed]
- SocaÃ
?a K, Doboszewska U, Szopa A, Serefko A, WÃ
?odarczyk M, et al. (2021) The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol Res 172: 105840.
[Crossref], [Google Scholar], [Indexed]
- Rutsch A, Kantsjö JB, Ronchi F (2020) The gut-brain axis: How microbiota and host inflammasome influence brain physiology and pathology. Front Immunol 11: 604179.
[Crossref], [Google Scholar], [Indexed]
- Góralczyk-BiÃ
?kowska A, Szmajda-Krygier D, KozÃ
?owska E (2022) The microbiota-gut-brain axis in psychiatric disorders. Int J Mol Sci 23: 11245.
[Crossref], [Google Scholar], [Indexed]
- Álvarez J, Fernández RJM, Guarner F, Gueimonde M, Rodríguez JM, et al. (2021) Gut microbes and health. Gastroenterol Hepatol 44: 519-535.
[Crossref], [Google Scholar], [Indexed]
- Dash S, Syed YA, Khan MR (2022) Understanding the role of the gut microbiome in brain development and its association with neurodevelopmental psychiatric disorders. Front Cell Dev Biol 10: 880544.
[Crossref], [Google Scholar], [Indexed]
- Osadchiy V, Martin CR, Mayer EA (2019) The gut-brain axis and the microbiome: Mechanisms and clinical implications. Clin Gastroenterol Hepatol 17: 322-32.
[Crossref], [Google Scholar], [Indexed]
- Averina OV, Zorkina YA, Yunes RA, Kovtun AS, Ushakova VM, et al. (2020) Bacterial metabolites of human gut microbiota correlating with depression. Int J Mol Sci 21: 9234.
[Crossref], [Google Scholar], [Indexed]
- Roth W, Zadeh K, Vekariya R, Ge Y, Mohamadzadeh M (2021) Tryptophan metabolism and gut-brain homeostasis. Int J Mol Sci 22: 2973.
[Crossref], [Google Scholar], [Indexed]
- Sun LJ, Li JN, Nie YZ (2020) Gut hormones in microbiota-gut-brain cross-talk. Chin Med J 133: 826-833.
[Crossref], [Google Scholar], [Indexed]
- Furness JB (2012) The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9: 286-294.
[Crossref], [Google Scholar], [Indexed]
- Wachsmuth HR, Weninger SN, Duca FA (2022) Role of the gut-brain axis in energy and glucose metabolism. Exp Mol Med 54: 377-392.
[Crossref], [Google Scholar], [Indexed]
- Pavlov VA, Tracey KJ (2012) The vagus nerve and the inflammatory reflex-linking immunity and metabolism. Nat Rev Endocrinol 8: 743-754.
[Crossref], [Google Scholar], [Indexed]
- Matteoli G, Boeckxstaens GE (2013) The vagal innervation of the gut and immune homeostasis. Gut 62: 1214-1222.
[Crossref], [Google Scholar], [Indexed]
- Thomas KR, Watt J, Wu CMJ, Akinrinoye A, Amjad S, et al. (2022) Pain and opioid-induced gut microbial dysbiosis. Biomedicines 10: 1815.
[Crossref], [Google Scholar], [Indexed]
- Wang F, Roy S (2017) Gut homeostasis, microbial dysbiosis and opioids. Toxicol Pathol 45: 150-156.
[Crossref], [Google Scholar], [Indexed]
- Holzer P (2009) Opioid receptors in the gastrointestinal tract. Regul Pept 155: 11-17.
[Crossref], [Google Scholar], [Indexed]
- Waterman SA, Costa M, Tonini M (1992) Modulation of peristalsis in the guinea-pig isolated small intestine by exogenous and endogenous opioids. Br J Pharmacol 106: 1004-1010.
[Crossref], [Google Scholar], [Indexed]
- Chakrabarti A, Geurts L, Hoyles L, Iozzo P, Kraneveld AD, et al. (2022) The microbiota-gut-brain axis: Pathways to better brain health. Perspectives on what we know, what we need to investigate and how to put knowledge into practice. Cell Mol Life Sci 79: 80.
[Crossref], [Google Scholar], [Indexed]
- Farzi A, Fröhlich EE, Holzer P (2018) Gut microbiota and the neuroendocrine system. Neurotherapeutics 15: 5-22.
[Crossref], [Google Scholar], [Indexed]
- Obermeier B, Daneman R, Ransohoff RM (2013) Development, maintenance and disruption of the blood-brain barrier. Nat Med 19: 1584-1596.
[Crossref], [Google Scholar], [Indexed]
- Kadry H, Noorani B, Cucullo L (2020) A blood-brain barrier overview on structure, function, impairment and biomarkers of integrity. Fluids Barriers CNS 17: 69.
[Crossref], [Google Scholar], [Indexed]
- Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV (2019) Blood-brain barrier: From physiology to disease and back. Physiol Rev 99: 21-78.
[Crossref], [Google Scholar], [Indexed]
- Daneman R, Prat A (2015) The blood-brain barrier. Cold Spring Harb Perspect Biol 7: a020412.
[Crossref], [Google Scholar], [Indexed]
- Profaci CP, Munji RN, Pulido RS, Daneman R (2020) The blood-brain barrier in health and disease: Important unanswered questions. J Exp Med 217: e20190062.
[Crossref], [Google Scholar], [Indexed]
- Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, et al. (2014) Intestinal permeability-a new target for disease prevention and therapy. BMC Gastroenterol 14: 189.
[Crossref], [Google Scholar], [Indexed]
- Vancamelbeke M, Vermeire S (2017) The intestinal barrier: A fundamental role in health and disease. Expert Rev Gastroenterol Hepatol 11: 821-834.
[Crossref], [Google Scholar], [Indexed]
- Thoo L, Noti M, Krebs P (2019) Keep calm: The intestinal barrier at the interface of peace and war. Cell Death Dis 10: 849.
[Crossref], [Google Scholar], [Indexed]
- Camilleri M (2019) Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 68: 1516-1526.
[Crossref], [Google Scholar], [Indexed]
- Leader G, Abberton C, Cunningham S, Gilmartin K, Grudzien M, et al. (2022) Gastrointestinal symptoms in autism spectrum disorder: A systematic review. Nutrients 14: 1471.
[Crossref], [Google Scholar], [Indexed]
- Goodwin MS, Cowen MA, Goodwin TC (1971) Malabsorption and cerebral dysfunction: A multivariate and comparative study of autistic children. J Autism Child Schizophr 1: 48-62.
[Crossref], [Google Scholar], [Indexed]
- Hsiao EY (2014) Gastrointestinal issues in autism spectrum disorder. Harv Rev Psychiatry 22: 104-111.
[Crossref], [Google Scholar], [Indexed]
- White JF (2003) Intestinal pathophysiology in autism. Exp Biol Med 228: 639-649.
[Crossref], [Google Scholar], [Indexed]
- Krigsman A, Walker SJ (2021) Gastrointestinal disease in children with autism spectrum disorders: Etiology or consequence? World J Psychiatry 11: 605-618.
[Crossref], [Google Scholar], [Indexed]
- Ashwood P, Anthony A, Torrente F, Wakefield AJ (2004) Spontaneous mucosal lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms: Mucosal immune activation and reduced counter regulatory interleukin-10. J Clin Immunol 24: 664-673.
[Crossref], [Google Scholar], [Indexed]
- Furlano RI, Anthony A, Day R, Brown A, McGarvey L, et al. (2001) Colonic CD8 and gamma delta T-cell infiltration with epithelial damage in children with autism. J Pediatr 138: 366-372.
[Crossref], [Google Scholar], [Indexed]
- Torrente F, Anthony A, Heuschkel RB, Thomson MA, Ashwood P, et al. (2004) Focal-enhanced gastritis in regressive autism with features distinct from crohn's and helicobacter pylori gastritis. Am J Gastroenterol 99: 598-605.
[Crossref], [Google Scholar], [Indexed]
- Walker SJ, Beavers DP, Fortunato J, Krigsman A (2016) A putative blood-based biomarker for autism spectrum disorder-associated ileocolitis. Sci Rep 6: 35820.
[Crossref], [Google Scholar], [Indexed]
- Alessandria C, Caviglia GP, Campion D, Nalbone F, Sanna C, et al. (2019) HLA-DQ genotyping, duodenal histology and response to exclusion diet in autistic children with gastrointestinal symptoms. J Pediatr Gastroenterol Nutr 69: 39-44.
[Crossref], [Google Scholar], [Indexed]
- Kantarcioglu AS, Kiraz N, Aydin A (2016) Microbiota-gut-brain axis: Yeast species isolated from stool samples of children with suspected or diagnosed autism spectrum disorders and in vitro susceptibility against nystatin and fluconazole. Mycopathologia 181: 1-7.
[Crossref], [Google Scholar], [Indexed]
- Srikantha P, Mohajeri MH (2019) The possible role of the microbiota-gut-brain-axis in autism spectrum disorder. Int J Mol Sci 20: 2115.
[Crossref], [Google Scholar], [Indexed]
- Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, et al. (2017) New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 5: p24.
[Crossref], [Google Scholar], [Indexed]
- Rose DR, Yang H, Serena G, Sturgeon C, Ma B, et al. (2018) Differential immune responses and microbiota profiles in children with autism spectrum disorders and co-morbid gastrointestinal symptoms. Brain Behav Immun 70: 354-368.
[Crossref], [Google Scholar], [Indexed]
- Bernier R, Golzio C, Xiong B, Stessman HA, Coe BP, et al. (2014) Disruptive CHD8 mutations define a subtype of autism early in development. Cell 158: 263-276.
[Crossref], [Google Scholar], [Indexed]
- Campbell DB, Buie TM, Winter H, Bauman M, Sutcliffe JS, et al. (2009) Distinct genetic risk based on association of MET in families with co-occurring autism and gastrointestinal conditions. Pediatrics 123: 1018-1024.
[Crossref], [Google Scholar], [Indexed]
- Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, et al. (2005) Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet 77: 265-279.
[Crossref], [Google Scholar], [Indexed]
- Weiss LA, Abney M, Cook EH, Ober C (2005) Sex-specific genetic architecture of whole blood serotonin levels. Am J Hum Genet 76: 33-41.
[Crossref], [Google Scholar], [Indexed]
- Costedio MM, Hyman N, Mawe GM (2007) Serotonin and its role in colonic function and in gastrointestinal disorders. Dis Colon Rectum 50: 376-388.
[Crossref], [Google Scholar], [Indexed]
- Manocha M, Khan WI (2012) Serotonin and GI disorders: An update on clinical and experimental studies. Clin Transl Gastroenterol 3: e13.
[Crossref], [Google Scholar], [Indexed]
- D'Eufemia P, Celli M, Finocchiaro R, Pacifico L, Viozzi L, et al. (1996) Abnormal intestinal permeability in children with autism. Acta Paediatr 85: 1076-1079.
[Crossref], [Google Scholar], [Indexed]
- de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, et al. (2010) Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr 51: 418-424.
[Crossref], [Google Scholar], [Indexed]
- Zhu S, Jiang Y, Xu K, Cui M, Ye W, et al. (2020) The progress of gut microbiome research related to brain disorders. J Neuroinflammation 17: p25.
[Crossref], [Google Scholar], [Indexed]
- Luczynski P, McVey NKA, Oriach CS, Clarke G, Dinan TG, et al. (2016) Growing up in a bubble: Using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int J Neuropsychopharmacol 19: pyw020.
[Crossref], [Google Scholar], [Indexed]
- Galea I (2021) The blood-brain barrier in systemic infection and inflammation. Cell Mol Immunol 18: 2489-2501.
[Crossref], [Google Scholar], [Indexed]
- Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, et al. (2009) Elevated immune response in the brain of autistic patients. J Neuroimmunol 207: 111-116.
[Crossref], [Google Scholar], [Indexed]
- Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, et al. (2010) Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry 68: 368-376.
[Crossref], [Google Scholar], [Indexed]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA (2005) Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 57: 67-81.
[Crossref], [Google Scholar], [Indexed]
- Garbett K, Ebert PJ, Mitchell A, Lintas C, Manzi B, et al. (2008) Immune transcriptome alterations in the temporal cortex of subjects with autism. Neurobiol Dis 30: 303-311.
[Crossref], [Google Scholar], [Indexed]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, et al. (2011) Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474: 380-384.
[Crossref], [Google Scholar], [Indexed]
- Fiorentino M, Sapone A, Senger S, Camhi SS, Kadzielski SM, et al. (2016) Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism 7: p49.
[Crossref], [Google Scholar], [Indexed]
- Novarino G, El-Fishawy P, Kayserili H, Meguid NA, Scott EM, et al. (2012) Mutations in BCKD-kinase lead to a potentially treatable form of autism with epilepsy. Science 338: 394-397.
[Crossref], [Google Scholar], [Indexed]
- Jagadapillai R, Qiu X, Ojha K, Li Z, El-Baz A, et al. (2022) Potential cross talk between autism risk genes and neurovascular molecules: A pilot study on impact of blood brain barrier integrity. Cells 11: p2211.
[Crossref], [Google Scholar], [Indexed]
- Rueda-Ruzafa L, Cruz F, Cardona D, Hone AJ, Molina-Torres G, et al. (2020) Opioid system influences gut-brain axis: Dysbiosis and related alterations. Pharmacol Res 159: p104928.
[Crossref], [Google Scholar], [Indexed]
- Thomas KR, Watt J, Wu CMJ, Akinrinoye A, Amjad S, et al. (2022) Pain and opioid-induced gut microbial dysbiosis. Biomedicines 10: p1815.
[Crossref], [Google Scholar], [Indexed]
- Wang F, Roy S (2017) Gut homeostasis, microbial dysbiosis and opioids. Toxicol Pathol 45: 150-156.
[Crossref], [Google Scholar], [Indexed]
- De Schepper HU, Cremonini F, Park MI, Camilleri M (2004) Opioids and the gut: Pharmacology and current clinical experience. Neurogastroenterol Motil 16: 383-394.
[Crossref], [Google Scholar], [Indexed]
- Koob GF, Maldonado R, Stinus L (1992) Neural substrates of opiate withdrawal. Trends Neurosci 15: 186-191.
[Crossref], [Google Scholar], [Indexed]
- Lee K, Vuong HE, Nusbaum DJ, Hsiao EY, Evans CJ, et al. (2018) The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacology 43: 2606-2614.
[Crossref], [Google Scholar], [Indexed]
- Brown JN, Ortiz GM, Angel TE, Jacobs JM, Gritsenko M, et al. (2012) Morphine produces immunosuppressive effects in nonhuman primates at the proteomic and cellular levels. Mol Cell Proteomics 11: 605-618.
[Crossref], [Google Scholar], [Indexed]
- Meng J, Yu H, Ma J, Wang J, Banerjee S, et al. (2013) Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS One 8: e54040.
[Crossref], [Google Scholar], [Indexed]
- Banerjee S, Sindberg G, Wang F, Meng J, Sharma U, et al. (2016) Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol 9: 1418-1428.
[Crossref], [Google Scholar], [Indexed]
- Whiteley P (2017) Food and the gut: Relevance to some of the autisms. Proc Nutr Soc 76: 478-483.
[Crossref], [Google Scholar], [Indexed]
- Reichelt KL, Hole K, Hamberger A, Saelid G, Edminson PD, et al. (1981) Biologically active peptide-containing fractions in schizophrenia and childhood autism. Adv Biochem Psychopharmacol 28: 627-643.
- Reichelt WM, Knivsberg AM, Nodland M, Stensrud M, Reichelt KL (1997) Urinary peptide levels and patterns in autistic children from seven countries and the effect of dietary intervention after four years. Dev Brain Dysfunct 10: 44-55.
- Reichelt KL, Tveiten D, Knivsberg AM, Bronstad G (2012) Peptides' role in autism with emphasis on exorphins. Microb Ecol Health Dis 23.
[Crossref], [Google Scholar], [Indexed]
- Shattock P, Kennedy A, Rowell F, Berney TP (1990) Role of neuropeptides in autism and their relationships with classical neurotransmitters. Brain Dysfunc 3: 328-345.
- Shanahan MR, Venturini AJ, Daiss JL, Friedman AE (2000) Peptide diagnostic markers for human disorders. European Patent Application 1-44.
- Sokolov O, Kost N, Andreeva O, Korneeva E, Meshavkin V, et al. (2014) Autistic children display elevated urine levels of bovine casomorphin-7 immunoreactivity. Peptides 56: 68-71.
[Crossref], [Google Scholar], [Indexed]
- Parashar A, Bhushan V, Mahanandia NC, Kumar S, Mohanty AK (2022) Non-SELEX method for aptamer selection against β-casomorphin-7 peptide. J Dairy Sci 105: 5545-5560.
[Crossref], [Google Scholar], [Indexed]
- Reichelt K, Ekrem J, Scott H (1990) Gluten, milk proteins and autism: Dietary intervention effects on behavior and peptide secretion. J Appl Nutr 42: 1-10.
- Knivsberg AM, Wiig K, Lind G, Nodland M, Reichelt KL (1990) Dietary intervention in autistic syndromes. Brain Dysfunct 3: 315-317.
[Crossref], [Google Scholar], [Indexed]
- Knivsberg AM, Reichelt KL, Nodland M, Hoien T (1995) Autistic syndromes and diet: A follow-up study. Scand J Educat Res 39: 223-236.
[Crossref], [Google Scholar]
- Lucarelli S, Frediani T, Zingoni AM, Ferruzzi F, Giardini O, et al. (1995) Food allergy and infantile autism. Panminerva Med 37: 137-141.
- Adams L, Conn S (1997) Nutrition and its relationship to autism. Foc Aut Dev Disabl 12: 53-58.
[Crossref], [Google Scholar]
- Knivsberg AM, Reichelt KL, Hoien T, Nodland M (2002) A randomised, controlled study of dietary intervention in autistic syndromes. Nutr Neurosci 5: 251-261.
[Crossref], [Google Scholar], [Indexed]
- Whiteley P, Haracopos D, Knivsberg AM, Reichelt KL, Parlar S, et al. (2010) The ScanBrit randomised, controlled, single-blind study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders. Nutr Neurosci 13: 87-100.
[Crossref], [Google Scholar], [Indexed]
- Ghalichi F, Ghaemmaghami J, Malek A, Ostadrahimi A (2016) Effect of gluten free diet on gastrointestinal and behavioral indices for children with autism spectrum disorders: A randomized clinical trial. World J Pediatr 12: 436-442.
[Crossref], [Google Scholar], [Indexed]
- Adams JB, Audhya T, Geis E, Gehn E, Fimbres V, et al. (2018) Comprehensive nutritional and dietary intervention for autism spectrum disorder-a randomized, controlled 12-month trial. Nutrients 10: p369.
[Crossref], [Google Scholar], [Indexed]
- Herbert MR, Buckley JA (2013) Autism and dietary therapy: Case report and review of the literature. J Child Neurol 28: 975-982.
[Crossref], [Google Scholar], [Indexed]
- Hsu CL, Lin CY, Chen CL, Wang CM, Wong MK (2009) The effects of a gluten and casein-free diet in children with autism: A case report. Chang Gung Med J 32: 459-465.
- https://doi.org/10.1176 /appi.books.9780890425787
- Winburn E, Charlton J, McConachie H, McColl E, Parr J, et al. (2014) Parents' and child health professionals' attitudes towards dietary interventions for children with autism spectrum disorders. J Autism Dev Disord 44: 747-757.
[Crossref], [Google Scholar], [Indexed]
- Elder JH, Shankar M, Shuster J, Theriaque D, Burns’ S, et al. (2006) The gluten-free, casein-free diet in autism: Results of a preliminary double blind clinical trial. J Autism Dev Disord 36: 413-420.
[Crossref], [Google Scholar], [Indexed]
- Seung H, Rogalski Y, Shankar M, Elder J (2007) The glutenand casein-free diet and autism: Communication outcomes from a preliminary double-blind clinical trial. Am J Speech Lang Pathol 15: 337-346.
- Johnson CR, Handen BL, Zimmer M, Sacco K, Turner K (2011) Effects of gluten free/casein free diet in young children with autism: A pilot study. J Dev Physical Disabil 23: 213-225.
- Hyman SL, Stewart PA, Foley J, Cain U, Peck R, et al. (2016) The gluten-free/casein-free diet: A double-blind challenge trial in children with autism. J Autism Dev Disord 46: 205-220.
[Crossref], [Google Scholar], [Indexed]
- Gonzalez-Domenech PJ, Diaz-Atienza F, Garcia Pablos C, Serrano Nieto S, Herreros Rodriguez O, et al. (2019) Influence of a gluten-free, casein-free diet on behavioral disturbances in children and adolescents diagnosed with autism spectrum disorder: A 3-month follow-up pilot study. J Ment Health Res Intellect Dis 12: 256-272.
[Crossref], [Google Scholar]
- Gonzalez-Domenech PJ, Diaz-Atienza F, Garca PC, Fernandez SML, Martinez-Ortega JM, et al. (2020) Influence of a combined gluten-free and casein-free diet on behavior disorders in children and adolescents diagnosed with autism spectrum disorder: A 12-month follow-up clinical trial. J Autism Dev Disord 50: 935-948.
[Crossref], [Google Scholar], [Indexed]
- Cekici H, Sanlier N (2019) Current nutritional approaches in managing autism spectrum disorder: A review. Nutr Neurosci 22: 145-155.
[Crossref], [Google Scholar], [Indexed]
- Mulloy A, Lang R, O'Reilly M, Sigafoos J, Lancioni G, et al. (2010) Gluten-free and casein-free diets in the treatment of autism spectrum disorders: A systematic review. Res Autism Spectr Disord 4: 328-339.
[Crossref], [Google Scholar]
- Gonzalez-Domenech PJ, Diaz-Atienza F, Gutierrez-Rojas L, Fernandez-Soto ML, Gonzalez-Domenech CM (2022) A Narrative review about autism spectrum disorders and exclusion of gluten and casein from the diet. Nutrients 14: p1797.
[Crossref], [Google Scholar], [Indexed]
- Piwowarczyk A, Horvath A, Lukasik J, Pisula E, Szajewska H (2018) Gluten- and casein-free diet and autism spectrum disorders in children: A systematic review. Eur J Nutr 57: 433-440.
[Crossref], [Google Scholar], [Indexed]
- Hyman SL, Stewart PA, Foley J, Cain U, Peck R, et al. (2016) The gluten-free/casein-free diet: A double-blind challenge trial in children with autism. J Autism Dev Disord 46: 205-220.
[Crossref], [Google Scholar], [Indexed]
- Mari-Bauset S, Llopis-Gonzalez A, Zazpe I, Mari-Sanchis A, Suarez-Varela MM (2016) Nutritional impact of a gluten-free casein-free diet in children with autism spectrum disorder. J Autism Dev Disord 46: 673-684.
[Crossref], [Google Scholar], [Indexed]
- Hediger ML, England LJ, Molloy CA, Yu KF, Manning-Courtney P, et al. (2008) Reduced bone cortical thickness in boys with autism or autism spectrum disorder. J Autism Dev Disord 38: 848-856.
[Crossref], [Google Scholar], [Indexed]
- Arnold GL, Hyman SL, Mooney RA, Kirby RS (2003) Plasma amino acids profiles in children with autism: Potential risk of nutritional deficiencies. J Autism Dev Disord 33: 449-454.
[Crossref], [Google Scholar], [Indexed]
- Elder JH, Kreider CM, Schaefer NM, de Laosa MB (2015) A review of gluten- and casein-free diets for treatment of autism: 2005-2015. Nutr Diet Suppl 7: 87-101.
[Crossref], [Google Scholar], [Indexed]
- Pedersen L, Parlar S, Kvist K, Whiteley P, Shattock P (2014) Data mining the ScanBrit study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders: Behavioural and psychometric measures of dietary response. Nutr Neurosci 17: 207-213.
[Crossref], [Google Scholar], [Indexed]
- Kumar PJ, O'Donoghue DP, Stenson K, Dawson AM (1979) Reintroduction of gluten in adults and children with treated coeliac disease. Gut 20: 743-749.
[Crossref], [Google Scholar], [Indexed]
- Daniloski D, McCarthy NA, Vasiljevic T (2021) Bovine β-casomorphins: Friends or foes? A comprehensive assessment of evidence from in vitro and ex vivo studies. Trends Food Sci Technol 116: 681-700.
[Crossref], [Google Scholar]
- Giribaldi M, Lamberti C, Cirrincione S, Giuffrida MG, Cavallarin L (2022) A2 milk and BCM-7 peptide as emerging parameters of milk quality. Front Nutr 9: 842375.
[Crossref], [Google Scholar], [Indexed]
- Trivedi MS, Shah JS, Al-Mughairy S, Hodgson NW, Simms B, et al. (2014) Food-derived opioid peptides inhibit cysteine uptake with redox and epigenetic consequences. J Nutr Biochem 25: 1011-1018.
[Crossref], [Google Scholar], [Indexed]
- Jyonouchi H, Geng L, Ruby A, Reddy C, Zimmerman-Bier B (2005) Evaluation of an association between gastrointestinal symptoms and cytokine production against common dietary proteins in children with autism spectrum disorders. J Pediatr 146: 605-610.
[Crossref], [Google Scholar], [Indexed]
- Vojdani A, O'Bryan T, Green JA, Mccandless J, Woeller KN, et al. (2004) Immune response to dietary proteins, gliadin and cerebellar peptides in children with autism. Nutr Neurosci 7: 151-61.
[Crossref], [Google Scholar], [Indexed]
- de Magistris L, Picardi A, Siniscalco D, Riccio MP, Sapone A, et al. (2013) Antibodies against food antigens in patients with autistic spectrum disorders. Biomed Res Int 2013: 729349.
[Crossref], [Google Scholar], [Indexed]
- Xu G, Snetselaar LG, Jing J, Liu B, Strathearn L, et al. (2018) Association of food allergy and other allergic conditions with autism spectrum disorder in children. JAMA Netw Open 1: e180279.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences