Growth and Tolerance with an Amino Acid Based Enteral Formula: A Two-year Retrospective Study from a Children's Rehabilitation Hospital
Amy Hulsey1, Pamela Cekola2*, Aimee Henrikson2, Heidi Reichert3, Sarah S Cohen3 and Krysmaru Araujo Torres2
1Department of Hospital Medicine, Bethany Children's Health Center, Bethany, OK, USA
2Department of Medical Affairs, Nestlé Health Science, Bridgewater, NJ, USA
3Epid Strategies A Division of ToxStrategies, Inc, Cary, NC, USA
*Corresponding author: Pamela Cekola, Nestle Health Science, Bridgewater, NJ, USA, Tel: +1 862-217-3001; E-Mail: pamela.cekola@us.nestle.com
Received date: August 17, 2022, Manuscript No. IPJCND-22-14347; Editor Assigned date: August 19, 2022, PreQC No. IPJCND-22-14347(PQ); Reviewed date: August 31, 2022, QC No. IPJCND-22-14347; Revised date: September 07, 2022, Manuscript No. IPJCND-22-14347(R); Published date: September 12, 2022, DOI: 10.36648/2472-1921.8.8.181
Citation: Hulsey A, Cekola P, Henrikson A, Reichert H, Cohen SS, et al. (2022) Growth and Tolerance with an Amino Acid Based Enteral Formula: A Two-year Retrospective Study from a Children’s Rehabilitation Hospital. J Clin Nutr Diet Vol.8 No.8:181
Abstract
Background: This study aimed to identify characteristics of a medically complex pediatric population that was fed an amino acid-based formula containing Medium-Chain Triglycerides (AA-MCT).
Methods: We examined calorie/protein prescription and intake, formula tolerance, concomitant use of Gastrointestinal (GI) medications, and growth (weight, height, Body Mass Index (BMI) z-scores). We reviewed medical records of children from a Hospital Rehabilitation Center, identifying those with a history of a tube-fed formula switch to the AA-MCT (n=23). We recorded demographic data (age, sex, diagnosis) and compiled outcomes data (formula intake, tolerance, GI medications, growth) at key time points over two years (pre-switch, at switch and post-switch).
Results: At AA-MCT initiation, mean age was 7.5 years and 48% of children had evidence of GI dysfunction. Atopic dermatitis and food allergies were present in 22%, others had genetic or metabolic disorders with severe GI impairment. Following switch to AA-MCT, mean calorie and protein intake increased, weight and height for age measures, and BMI z-scores were within expected ranges for most children. A reduction in GI medications was observed for some after the switch.
Conclusions: Children with medical complexity switched to AA-MCT formula demonstrated growth. Formula was well tolerated, as shown by increased intake of formula, appropriate growth for age and BMI z-scores up to 1 year post-switch.
Keywords: Amino acid formula; Pediatric nutrition; Medically complex child; Enteral nutrition; Gastrointestinal impairment; Malabsorption; Cow’s milk protein allergy.
Acronyms: AAF: Amino acid-based formula; AA-MCT: Amino acid-based formula, Alfamino® Junior; AAP: American academy of pediatrics; BMI: Body max index; CMPA: Cow’s milk protein allergy; CP: Cerebral palsy; EH: Extensively hydrolyzed; EoE: Eosinophilic esophagitis; ESPGHAN: European society for pediatric gastroenterology hepatology and nutrition; FPIES: Food protein-induced enterocolitis; GERD: Gastroesophageal reflux disease; GI: Gastrointestinal; MCT: Medium-chain triglycerides; WHO: World health organization.
Introduction
In the United States, an estimated 0.4%-0.7% of children (320,000 to 560,000) are considered medically complex; these children account for 15%-33% of pediatric healthcare costs [1]. Improving care management for these patients is critical to reducing healthcare costs. As well, optimal medical care including well-tolerated nutrition that is complete and balanced is key to growth, development and quality of life. Amino acid-based nutritional formulas were originally developed for infants with formula intolerance due to Cow’s Milk Protein Allergies (CMPA). More recently, use of amino acid-based formulations has been recommended for some infants and children with medically complex conditions that affect nutrient absorption and utilization, i.e., conditions of genetic, metabolic, and neurologic origin.
Clinical practice guidelines now specifically recommend amino acid-based feeding formulations beyond infancy for children with Cow’s Milk Protein Allergy (CMPA), Eosinophilic Esophagitis (EoE) and Food Protein-Induced Entercolitis Syndrome (FPIES) [2-13]. Some children have malabsorptive or allergic conditions that make them sensitive to intact protein or even to extensively hydrolyzed protein beyond the first year of life [5]. For such children over the age of 12 months, guidelines from the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) and the American Academy of Pediatrics (AAP) recommend continued use of amino acid-based formulas to support growth and development in children with CMPA, including those who present with gastroesophageal reflux disease and symptoms such as dyspepsia and abdominal pain [5,14].
Children with a wide range of genetic and metabolic disabilities also suffer from feeding difficulties and experience gastrointestinal symptoms that interfere with nutritional intake and adversely affect nutritional status, resulting in growth failure and decreased quality of life [15,16]. Amino acid-based formulations are prescribed for children with genetic disorders such as DiGeorge syndrome, chronic transaminases and metabolic disorders that hamper tolerance of formulas with intact or hydrolyzed proteins [17,18].
In addition, children with neurological impairment account for about 28% of all children with medical complexity [19]. It is estimated that up to 80%-90% of these children have some type of feeding disorder that is caused by GastroEsophageal Reflux Disease (GERD) with or without aspiration, dysphagia, dysmotility and constipation that can interfere with oral intake, and necessitating enteral tube feeding [15,16,20,21]. Clinical guidelines support the use of enteral tube feeding for children with neurological impairment. Polymeric enteral formulas are recommended for most children, but protein hydrolysates and amino-acid based formulas are advised for children who respond poorly to standard formula [22-25]. Despite the use of amino acid-based enteral formulas in more severely compromised children, there is a gap in identifying all children, regardless of disease severity, who could benefit from an amino acid based enteral tube feeding. A recent survey of clinicians and family caregivers identified feeding tolerance and formula selection as the top ranked research priority for children with neurological impairment [19].
The current study was a retrospective assessment of children who were switched to a specialized nutritionally complete hypoallergenic formula while staying at a Children's Rehabilitation Hospital. The formula was Amino-Acid based and contained Medium Chain Triglycerides (AA-MCT), a formulation developed to be well tolerated and contain readily absorbed macronutrients. The study population included children over the age of 12 months who had medically complex conditions and needed enteral tube feeding. The primary aim of this study was to describe the population of medically complex children fed AA-MCT.
Methods
Study design
This study was a retrospective review of medical records for children who stayed in a Children’s hospital rehabilitation center in Bethany, Oklahoma (USA) and were switched by their healthcare professional to the AA-MCT formula. Records were eligible for study inclusion if the patient was over the age of 12 months and under 19 years. Demographic data were compiled, and outcomes data (nutritional status, feeding tolerance, GI medication use, growth) were collected at five time points over two years (12 months pre-switch to AA-MCT formula, 6 months pre-switch, at switch, and at 6 and 12 months post-switch).
Nutritional formula
The intervention was a nutritionally complete formula (Alfamino® Junior, unflavored, Nestlé Healthcare Nutrition, Bridgewater, NJ, USA) that contained protein in the form of free Amino Acids (AA) with fat primarily as readily absorbed Medium-Chain Triglycerides (MCT). The Alfamino® Junior formula (AA-MCT) contains complete and balanced micronutrients, including vitamins and minerals, provides 30 kcals per ounce when mixed as directed and was developed for management of multiple food allergies, eosinophilic GI disorders or malabsorptive conditions. AA-MCT is recommended for use in children 1 to 13 years of age (Table 1).
| Nutrient | Source |
|---|---|
| Protein source | Amino acids |
| Protein (g/100 kcal, % kcal) | 3.3 / 13% |
| Fat source | Medium chain triglycerides, soybean oil |
| Fat (g/100 kcal, % kcal) | 4.4 / 38% |
| MCT, % of fat | 65% |
| Carbohydrate source | Corn syrup solids, potato starch |
| Carbohydrate (g/100 kcal, % kcal) | 12.2 / 49% |
Table 1: Macronutrient composition of the nutritionally complete AA-MCT enteral formula for children 1-13 years of age who have food allergies or other conditions associated with nutrient intolerance or malabsorption.
Outcome measures
The primary objective of this retrospective study was to describe characteristics of the population using the AA-MCT formula, including subject demographics (age, sex, ethnicity); primary and secondary diagnoses and medical conditions that were indications for use of the AA-MCT formula.
Secondary outcomes, collected as available, were growth (weight and height), achievement of nutritional goals (average intake of formula consumed over 7 days, at each time point), feeding tolerance (vomiting, flatulence, stool frequency and consistency), serum markers related to nutritional status (vitamin D, albumin, phosphorus, calcium, magnesium) and GI medication use (proton pump inhibitor, H2 blocker, prokinetic agent, anti-diarrheal, fiber supplement, laxatives, stool softeners, antiemetic, other). These measures were compiled for time points 12 and 6 months prior to transition to AA-MCT formula, at the time of the formula transition and then assessed again at 6 and 12 months while AA-MCT formula intake continued.
Ethics and informed consent
The study was conducted in accordance with the declaration of Helsinki and the study protocol was submitted and approved by WCG IRB, Puyallup, Washington, USA. This study was registered with clinical trials.gov (ID# NCT03497091). A Health Insurance Portability Accountability Act (HIPAA) waiver of consent was allowed because patients were retrospectively identified via medical records.
Statistics
This study was entirely descriptive in nature. Descriptive statistics were computed for continuous measures as means, standard deviations, medians, minimums, and maximums and for categorical measures as counts and percentages. Measures were summarized at the time points of interest (12 and 6 months pre-switch, at switch and 6 and 12 months post-switch) using all available data from the medical records. At this institution, anthropometric measures are plotted on either World Health Organization (WHO) growth standards or reference curves with a focus on cerebral palsy population using gender and gross motor function measures and these standards were used to compute weight and height percentiles along with weight-for-age and height-for-age z-scores, BMI z-scores and change in BMI z-scores [26-28]. All statistical analyses were performed using Stata Statistical Software: (Release 15, College Station, TX: StataCorp LLC).
Results
Primary objective and outcomes
The primary objective of this retrospective study was to describe the pediatric population with a history of use with AA-MCT formula. Results include:
Demographics
The medical records of 26 children, aged 1 to 18 years, were identified, and reviewed; 3 subjects were excluded due to insufficient pre or post data, leaving 23 subjects for analysis. At initiation of the AA-MCT formula, the mean age of the children was 7.5 ± 5.4 years, 61% were female, multiple ethnicities were represented (48% Caucasian, 35% African American, 13% American Indian/Alaska native, 4% Asian), and 100% were tube fed (70% via gastrostomy [G], 30% via gastrojejunostomy [G/J] tubes).
Diagnoses and indications for use of AA-MCT
A variety of primary diagnoses were observed, which facilitated a better understanding of why the children were being fed an amino acid-based formula. Almost half of the children (48%) presented with a primary diagnosis of gastrointestinal disorder, which included atrophic gastritis, delayed gastric motility, diarrhea, flatulence, gastroschisis and vomiting. Other primary disorders (17%) included: DiGeorge syndrome, chronic transaminitis, failure to thrive and other metabolic diseases/disorders. Feeding disorders and atopic dermatitis each accounted for 13%, respectively. Allergies to milk, soy, wheat, peanuts, egg, and red dye were present in 9% of the population.
To enhance understanding of the patient conditions and to clarify rationale for enteral tube feeding of AA-MCT, major secondary diagnoses were also documented. These diagnoses/conditions were gastrostomy (100%), constipation (61%), anoxic brain damage or injury (39%), feeding difficulties (35%), dysphagia (22%), epilepsy (17%), gastroesophageal reflux disease (13%), abdominal distention (9%), calculus of kidney (9%), calcium metabolism disorder (9%), bone density disorder (9%), quadriplegic infantile cerebral palsy (9%), and spastic quadriplegic cerebral palsy (9%).
Secondary objectives and outcomes
Secondary objectives included achievement of nutritional goals, enteral feeding prescription and intake, growth, tolerance, GI medication use, hospitalization and readmittance and serum markers. Results included:
Achievement of nutritional goals
Enteral feedings (continuous and bolus) provided most of the calories and protein needed for children with these medically complex conditions. Additional forms of nutrition (oral intake, parenteral nutrition) were documented to establish the full scope of nutrition support received. Oral intake was prescribed in one subject at 12 months prior to switch to AA-MCT; no other oral intake was prescribed. One subject received supplemental parenteral nutrition at alltime points, while most other patients received one or more modulars such as protein supplementation and Vitamin D3. Prior to switching to AA-MCT, enteral formula use varied; the most common formula children received was another amino acid formula (for children aged 1+ year), followed by standard pediatric formulas, and then extensively hydrolyzed formulas.
Feeding prescription and actual intake
In the year prior to the switch to the AA-MCT formulation, lower calorie/protein prescription and intake was noted in more than half of the children (60%). Upon switching to the AA-MCT, intake for calories and protein increased for all children, and the increases were sustained in most of the children (n=20) at 6 months post-switch. Although only 15 children continued enteral feeding at 12 months, these children continued to have increases in both calorie and protein prescriptions and intake while on the AA-MCT (Table 2). All children were tube-fed (70% G-tube, 30% G/J-tube), and the feeding strategy before and after switch was about equally divided between continuous and bolus feedings.
| -12 Months | -6 Months | Switch | +6 Months | +12 Months | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean [SD] | Mean [SD] | Mean [SD] | Mean [SD] | Mean [SD] | ||||||
| Median | Median | Median | Median | Median | ||||||
| RX | Intake | RX | Intake | RX | Intake | RX | Intake | RX | Intake | |
| Subjects (N) | 14 | 14 | 15 | 15 | 23 | 23 | 20 | 20 | 15 | 15 |
| Calorie, | 892 [324] | 841 [315] | 839 [290] | 620 [197] | 829 [252] | 777 [256] | 950 [292] | 860 [255] | 970 [321] | 900 [297] |
| kcal/day | 930 | 902 | 733 | 635 | 800 | 732 | 943 | 855 | 960 | 915 |
| Protein | 36 [13] | 36 [16] | 32 [12] | 21 [9] | 32 [10] | 26 [8] | 37 [11] | 28 [8] | 41 [15] | 30 [10] |
| g/day | 40 | 33 | 32 | 20 | 32 | 24 | 34 | 27 | 37 | 30 |
Table 2: Formula* prescription and Intake over the study period, all subjects, N=23.
*Combined feed includes enteral formula, parenteral nutrition, complementary nutrition sources, and nutrition modular; RX=prescription.
Overall, intake of calorie and protein goals was achieved. The mean percentage of daily intake at switch to AA-MCT was 100% for calories and 99% for protein. At one-year post-switch, 99% of calorie and 99% of protein goals were maintained (Table 3).
| -12 Months | -6 months | Switch | +6 months | +12 months | |
|---|---|---|---|---|---|
| Mean [SD] | Mean [SD] | Mean [SD] | Mean [SD] | Mean [SD] | |
| Median | Median | Median | Median | Median | |
| (Min,Max) | (Min,Max) | (Min,Max) | (Min,Max) | (Min,Max) | |
| Subjects (N) | 14 | 15 | 23 | 20 | 15 |
| Calorie, % | 95 [19.1] | 87 [17.8] | 100 [11.7] | 99 [8.5] | 99 [7.6] |
| 96 | 97 | 98 | 99 | 98 | |
| (69, 136) | (54, 106) | (86, 139) | (82, 121) | (88, 118) | |
| Protein, % | 99 [24.7] | 94 [47.6] | 99 [15.6] | 99 [9.2] | 99 [6.9] |
| 98 | 96 | 99 | 99 | 99 | |
| (63, 150) | (51, 244) | (53, 140) | (82, 121) | (87, 118) |
Table 3: Percentage of estimated daily calorie and protein met by formula* intake over study period, all subjects, N=23. * Combined feed includes enteral formula, parenteral nutrition, complementary nutrition sources and nutrition modular.
Growth
Weight and height percentiles along with weight-for-age and height-for-age z-scores, BMI z-scores and change in BMI z-scores were reported. Overall, adequate growth was achieved, evidenced by appropriate increases in weight-for-age and height-for-age at both 6 and 12 months post-switch for both boys and girls across all age groups (Table 4).
| -12 Months | -6 months | Switch | +6 months | +12 months | |
|---|---|---|---|---|---|
| N=14 | N=16 | N=23 | N=21 | N=16 | |
| N | N | N | N | N | |
| Mean [SD] | Mean [SD] | Mean [SD] | Mean [SD] | Mean [SD] | |
| Median | Median | Median | Median | Median | |
| (Min,Max) | (Min,Max) | (Min,Max) | (Min,Max) | (Min,Max) | |
| Overall | |||||
| Weight, kg | 14 | 16 | 23 | 20 | 15 |
| 21.5 [9.8] | 22.2 [9.6] | 21.1 [11.3] | 23.2 [9.7] | 25.6 [8.0] | |
| 23 | 23.6 | 21.3 | 24 | 26.2 | |
| (4.7, 37.7) | (7.7, 40.5) | (7.0, 47.4) | (9.6, 40.5) | (12.9, 35.5) | |
| Height, cm | 14 | 16 | 23 | 20 | 15 |
| 108.8 [2726.8] | 109.4 [24.0] | 103.5 [25.1] | 110.0 [23.2] | 117.0 [19.1] | |
| 113.5 | 118 | 113 | 121 | 121 | |
| (58.0, 142.0) | (68.0, 142.0) | (64.5, 142.0) | (73.8. 142.0) | (84.0, 145.0) | |
| Boys | |||||
| Weight, kg | 6 | 6 | 9 | 9 | 7 |
| 24.5 [6.2] | 26.0 [5.5] | 25.7 [11.7] | 26.4 [9.4] | 29.0 [5.3] | |
| 23.5 | 24.1 | 25.9 | 25.5 | 27 | |
| (17.5, 34.0) | (19.8, 34.0) | (7.5, 47.4) | (9.6, 40.5) | (23.5, 35.5) | |
| Height, cm | 6 | 6 | 9 | 9 | 7 |
| 119.7 [12.0] | 121.5 [10.9] | 114.1 [22.8] | 118.3 [18.6] | 128.1 [9.1] | |
| 117 | 119.5 | 118 | 122 | 127 | |
| (109.0, 142.0) | (112.0, 142.0) | (67.0, 142.0) | (73.8, 142.0) | (120.0, 145.0) | |
| Girls | |||||
| Weight, kg | 8 | 10 | 14 | 11 | 8 |
| 19.2 [11.7] | 19.9 [11.0] | 18.1 [10.3] | 20.6 [9.6] | 22.7 [9.0] | |
| 22.5 | 22.8 | 13 | 17.1 | 21.4 | |
| (4.7, 37.7) | (7.7, 40.5) | (7.0, 42.0) | (10.5, 37.5) | (12.9, 35.0) | |
| Height, cm | 8 | 10 | 14 | 11 | 8 |
| 100.8 [32.5] | 102.2 (27.2) | 96.7 [24.9] | 103.1 [25.1] | 107.1 [20.6] | |
| 112 | 113.8 | 85.4 | 88.7 | 104.2 | |
| (58.0, 141.0) | (68.0, 130.0) | (64.5, 132.0) | (77.0, 135.0) | (84.0, 132.0) | |
Table 4: Weight and height over the study period, all subjects combined and stratified by sex at switch, N=23.
Similarly, height-for-age mean percentiles were 47% and 51% (CP growth charts, N=18); 23%, and 9th (WHO growth charts, N=5, N=1, respectively), as shown in Table 5.
Weight-for-age mean percentiles at switch and 12 months post-switch were 57% and 60%, respectively, for children on CP Growth Charts (N=18) and were 28% and 33% for children on WHO Growth Charts (N=5), as shown in Table 5.
| -12 months | -6 months | Switch | +6 months | +12 months | |
|---|---|---|---|---|---|
| N | N | N | N | N | |
| Mean [SD] | Mean [SD] | Mean [SD] | Mean [SD] | Mean [SD] | |
| Median | Median | Median | Median | Median | |
| [Min, Max] | [Min, Max] | [Min, Max] | [Min, Max] | [Min, Max] | |
| Weight-for-age z-score | 14 | 16 | 23 | 20 | 15 |
| -0.3 [0.8] | 0.01 [0.6] | -0.7 [1.2] | 0.1 [0.8] | 0.2 [0.6] | |
| -0.3 | -0.02 | 0.1 | 0.1 | 0.1 | |
| (-1.5, 0.9) | (-0.9, 1.0) | (-2.8, 2.3) | (-1.6, 1.5) | (-1.0, 1.2) | |
| Height-for-age z-score | 14 | 16 | 23 | 20 | 15 |
| -0.3 [1.1] | -0.2 [1.2] | -0.6 [1.7] | -0.4 [1.2] | -0.1 [0.6] | |
| 0.1 | -0.06 | -0.4 | -0.2 | 0.2 | |
| (-2.9, 0.8) | (-2.8, 1.3) | (-4.6, 1.5) | (-3.8, 1.3) | [-1.4, 1.1] | |
| BMI z-score | 14 | 16 | 23 | 20 | 15 |
| -0.20 [1.03] | 0.08 [1.17] | 0.47 [1.19] | 0.59 [1.32] | 0.23 [1.48] | |
| -0.32 | 0.23 | 0.68 | 0.66 | 0.08 | |
| (-1.71, 1.81) | (-2.27, 1.92) | (-2.58, 3.30) | (-1.39, 3.00) | (-2.28, 3.08) | |
| CP Growth Charts (N=18) | |||||
| Weight-for-age | 12 | 14 | 18 | 17 | 14 |
| percentile | 48 [24] | 53 [21] | 57 [28] | 58 [23] | 60 [20] |
| 42 | 53 | 57 | 59 | 56 | |
| (14, 82) | (18, 83) | (3, 99) | (18, 93) | (17, 89) | |
| Height-for-age | 12 | 14 | 18 | 17 | 14 |
| percentile | 48 [26] | 47 [28] | 47 [29] | 49 [27] | 51 [19] |
| 56 | 48 | 51 | 51 | 58 | |
| (1, 79) | (1, 91) | (1, 89) | (11, 91) | (12, 86) | |
| WHO Growth Charts (N=5) | |||||
| Weight-for-age | 2 | 2 | 5 | 3 | 1 |
| percentile | 7 [0.9] | 34 [22] | 28 [31] | 37 [27] | 33 |
| 7 | 34 | 19 | 49 | ||
| (7, 8) | (18, 49) | (0.2, 61) | (6, 56) | ||
| Height-for-age | 2 | 2 | 5 | 3 | 1 |
| percentile | 34 [48] | 45 [63] | 23 [40] | 4 [5] | 9 |
| 34 | 45 | 2 | 2 | ||
| (0.2, 67) | (0.3, 90) | (0, 93) | (0.01, 9) | ||
Table 5: Growth measures (Z-scores, CP and WHO growth chart percentiles).
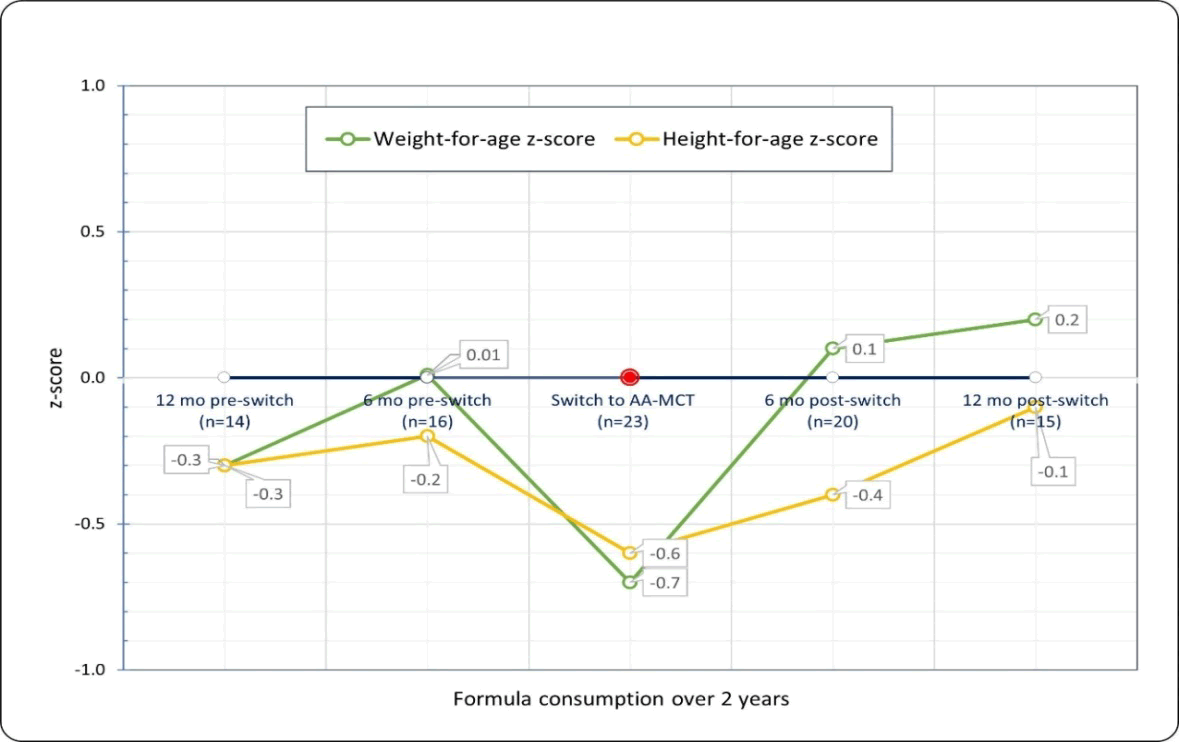
Across age groups, children showed positive growth trajectory from time-of-switch to 12 months post-switch. Mean z-scores for weight-for-age were -0.07 [SD 1.2] at switch and 0.2 [0.6] at 12 months post-switch, with z-scores for height-for-age of -0.6 [1.7] and -0.1 [0.6] (Figure 1).
Based on mean BMI z-scores of 0.47 [SD 1.19] at switch, 0.59 [1.32] at 6 months post-switch and 0.23 [1.48] at 12 months post-switch (Table 5), along with mean change in BMI z-scores of -0.08 [SD 0.95; N=20] from switch to 6 months post-switch and -0.43 [1.01; N=15] from switch to 12 months post-switch, malnutrition was not present in the study population. The trend in BMI z-scores at 12 months post-switch remained in an appropriate range, although interpretation may be limited by the reduced sample size. The change in BMI z-scores, at both 6 months and 12 months post-switch, indicated there was no growth faltering in this population.
Tolerance
Over time there were no significant changes in GI tolerance events. Upon switching, the AA-MCT was generally well tolerated; there was only one reported episode of the tube feeding briefly held due to short-term intolerance. No nausea was reported at switch and most children (91.3%) did not experience flatulence. Four subjects with a primary diagnosis of vomiting continued to report episodes. Prior to the switch, the reported frequency of vomiting was of 23.5%. During AA-MCT consumption, 20%-25% of subjects reported vomiting, with a mean of 3-5 episodes per subject. There were no reports of formula discontinuation post-switch. The number of stools per child per day were a mean of 2.1 [SD 1.1] at switch as compared to 1.6 [1.1] stools per day at 6 months prior to initiation of the AA-MCT formulation. Stools per day decreased to 1.5 [0.9] at 6 months post-switch and 1.6 [1.2] at 12 months post-switch, with consistently soft stool consistency.
GI medication use
In children with medically complex conditions, GI medications use is common and accordingly, we observed use of GI medications by all children in this study at all-time points [29]. Throughout the 2-year review, the mean number of GI medications per subject, ranged from 1.6 to 2.6. At switch to AA-MCT formula, the children (n=23) received a mean of 2.3 [1.2] GI medications. The most frequent GI medications prescribed were laxatives (40%), proton pump inhibitors (15%), antiemetics (15%), H2 blockers (11%), prokinetic agents (6%), and stool softeners (4%). At 1 year post formula switch, 25% (4 of 16) of children were prescribed an additional medication, 44% had no change in GI medications, and 31% had a reduction in 1 or more GI medications.
Hospitalizations and readmittance
The population studied was medically complex; children had conditions that brought them back to the facility after discharge. We reviewed all children who were discharged and readmitted at some time point over the two-year review. Data were not sufficient to show any specific pattern or difference between pre and post-switch to AA-MCT feeding formula.
Serum markers
Mean values for vitamin D, albumin, phosphorus, calcium and magnesium, serum markers were within normal ranges at alltime points, thus indicating that electrolyte imbalance, metabolic complications, or re-feeding syndrome were unlikely (Table 6).
| -12 months | -6 months | Switch | +6 months | +12 months | Site normal values | |
|---|---|---|---|---|---|---|
| N | N | N | N | N | ||
| Mean | Mean | Mean | Mean | Mean | ||
| Vitamin D (ng/mL) | 2 | 9 | 14 | 12 | 8 | 30-100 ng/mL |
| 33.5 | 40.2 | 45.6 | 47.3 | 49 | ||
| Albumin (g/dL) | 1 | 12 | 17 | 17 | 12 | 3.6-5.1 g/dL |
| 4.5 | 3.9 | 4 | 3.9 | 4.1 | ||
| Phosphorous (mg/dL) | 1 | 7 | 11 | 10 | 10 | 3-6 mg/dL (age specific) |
| 3.9 | 4.4 | 4.9 | 4.4 | 4.6 | ||
| Calcium (mg/dL) | 1 | 12 | 17 | 18 | 11 | 8.5-10.6 mg/dl |
| 10.2 | 9.5 | 9.5 | 9.6 | 9.5 | ||
| Magnesium (mEq/L) | 1 | 6 | 10 | 9 | 6 | 1.5-2.5 mg/dL |
| 1.9 | 2.1 | 2.1 | 2.1 | 2.1 |
Table 6: Serum Markers.
Although not an accurate parameter for nutritional status, albumin was normal, indicating no protein losses or acute inflammation. Vitamin D was also within normal range. These findings suggested positive feeding tolerance and adequate absorption of nutrients.
Discussion
Study findings in perspective
In infants and young children, feeding-formula intolerance has long been attributed to allergies [2,13,30-34]. It is now recognized that such intolerance may also be associated with genetic, metabolic, neurologic, and developmental conditions in which gastrointestinal dysfunction is prominent [15,17,20,23]. Further, children experience gastrointestinal dysfunction with intolerance of feedings far beyond infancy, so nutritional adequacy must be assured for childhood growth [8,23,35,36]. In this descriptive study of children 1 to 18 years-old, we identified a variety of medical conditions associated with the use of an amino acid-based enteral formula with medium-chain triglycerides. Although this formula was originally developed for children with moderate to severe allergic conditions (CMPA, complex food allergies, eosinophilic GI disorders and severe malabsorptive conditions), our current study findings highlighted benefits for children with a wide range of other complex medical conditions that may lead to severe feeding difficulties and to shortfalls in growth and development.
Our study examined health and growth outcomes up to 1 year after feeding switch to AA-MCT. The formula was well tolerated in this population of children with medically complex conditions. Nutritional intake (calories and protein) remained consistent over the switch, highlighting that nutrition goals continued to be met and the AA-MCT formula effectively supported growth, as evidenced by increased weight and height that was paralleled by improved z-scores for weight and height. Such improvements were sustained up to 12 months post-switch. BMI z-scores were consistently in an appropriate reference standard range indicating no malnutrition and change in BMI z-scores further described no growth faltering from switch to AA-MCT up to 12 months post-switch [35,36].
This was a very complex group of pediatric cases with multiple medical conditions and symptoms like those seen with feeding intolerance (nausea, vomiting). Results show that the existing symptoms were not exacerbated by AA-MCT and that feedings were not stopped due to intolerance. Stools remained consistent in number and softness. Serum markers were within normal ranges suggesting adequate nutrient absorption and feeding tolerance. Gastrointestinal medication use was common up to 1 year post switching to AA-MCT, and some children (31%) showed a reduction of 1 or more GI medications.
Relevant studies and nutritional guidelines
The use and efficacy of Amino Acid-based Formula (AAF) has been studied in both healthy and chronically ill infants and children. A recent prospective, multi-center study demonstrated that both Extensively Hydrolyzed (EH) formulas and AAF were well tolerated and promoted normal growth in healthy term infants; there were no differences in the children’s growth rates for weight or length between AAF and EH formulas during the study [37]. The use of AAF has also been studied in children with other chronic conditions, including severe allergies; results demonstrated safety and efficacy of AAF in nutritional management of these children (Table 7).
| Author, year | Condition | Study design | Results & conclusions |
|---|---|---|---|
| Nocerino et al., 2021 [3] | IgE-mediated cow’s milk protein allergy (CMPA) | Double-blind, placebo controlled prospective trial with 29 patients aged 1-36 months to evaluate hypoallergenicity of an Amino Acid-based Formula (AAF). | The AAF was well tolerated by children with CPMA. These results support the use of AAF as a dietary option for non-breastfed children with CMPA. |
| Vandenplas et al., 2021 [38] | CMPA | Prospective, single-center, study of growth parameters in a large cohort of non-breastfed Chinese infants with challenge-confirmed CMPA (n=218 with confirmed CMPA, median age 16.1 weeks on enrollment) who were fed AAF for 9 months, in conjunction with a CMPA-free complementary diet. | AAF supported normal growth over 9 months in a cohort of Chinese infants with challenge-confirmed non-IgE-mediated CMPA. Growth was monitored by determining weight-for-age, length for age, and head circumference for age Z scores. |
| Cekola et al., 2021 [39] | CMPA malabsorptive conditions | Prospective, multicenter, post-market surveillance program of 144 infants (<12 months of age) to assess tolerance and prevalence of adverse effects resulting from AAF intake. | There were no safety concerns in infants with CMPA, severe CMPA and malabsorptive conditions receiving AAF. A high degree of caregiver satisfaction was identified with the use of the formula. |
| Fierro et al., 2020 [40] | CMPA | Phase III/IV prospective, multicenter, open-label, international study in 30 infants and children with immunoglobulin E-mediated CMPA consuming an AAF formula for at least 7 days. | The formula met the American Academy of Pediatrics criteria for hypoallergenicity and was well tolerated with no signs of allergic reaction. |
| Atwal et al., 2019 [7] | EoE | Narrative literature review to explore the effectiveness of AAF for the management of children with EoE. | Based on the results of 10 studies, children receiving AAF formula had reduced eosinophil levels with 75%-100% experiencing improvement or resolution of clinical symptoms vs. remission rates of 75%-81% and 40%-69% for Empirical Elimination Diet (EED) and Targeted Elimination Diet (TED) respectively. |
| Canini et al., 2017 [41] | CMPA | Multi-center clinical trial of children with CMPA randomized to 2 groups to receive AAF or EH whey formula and compared to healthy controls receiving follow-on or growing up formulas to assess body growth and protein metabolism. | At the end of the 12-month study, the weight z-scores and protein metabolism were similar between groups without significant differences. The use of AAF in children with CMPA (ages 5-12 months) supports normal body growth with no alterations in protein metabolism. |
| Vanderhoof et al., 2016 [42] | CMPA | Prospective, observational study of 30 infants (age 1-12 months) with history of weight loss and persistent allergic response while on EH formulas were transitioned to an AAF for 12 weeks. | At 12 weeks, the mean weight (z-score change) improved+0.43 ± 0.28 (mean ± standard deviation). Improvements in allergic symptoms including atopic dermatitis were noted. The use of an AAF in infants with CMPA supported weight gain and improvement in allergic symptoms. |
Table 7: Clinical studies on conditions for which use of amino acid-based feeding formula is indicated. CMPA, cow’s milk protein allergy; AAF, amino acid-based formula; EH, extensively hydrolyzed; EoE, Eosinophilic esophagitis.
Practice guidelines from professional society’s further support the use of AAF in the management of chronic conditions including CMPA, food allergies, FPIES, neurologic impairment, EoE and DiGeorge syndrome (Table 8).
| Author, year | Condition | Expert opinion | Recommendations |
|---|---|---|---|
| Vanderplas et al., 2021 [2] | CMPA | Review of current guidelines for the management of CMPA | The use of AAF is recommended for children with severe CMPA. Possible use as a diagnostic elimination diet prior to diagnostic challenge. |
| Gargano et al., 2021 [30] | Food allergies (IgE-mediated, Mixed IgE/non IgE mediated, non-IgE mediated) | Narrative review of immune and non-immune adverse reactions to food | The use of AAF may be required in children with CMPA during the first years of life when severe symptoms persist (i.e., severe gastrointestinal bleeding, anaphylaxis). |
| Nowak-Wegrzyn et al., 2017 [13] | Food protein-induced enterocolitis syndrome (FPIES) | International evidence-based consensus guidelines for diagnosis and management of FPIES | AAF is recommended for children who do not tolerate extensively hydrolyzed formulas and for those with Failure to Thrive. |
| Romano et al., 2017 [22] | Neurologic impairment | European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) guidelines for evaluation and treatment of GI and nutritional complications in children with neurologic impairment | Most children should tolerate a polymeric diet, but some may need a semi-elemental or elemental formula. Casein hydro lysates and amino acid-based formulas may be indicated for use in some patients. |
| Papadopoulow et al., 2014 [10] | EoE | Eosinophilic esophagitis working group and gastroenterology committee of ESPGHAN position paper | AAF is recommended for use in treating children with EoE who have multiple food allergies, Failure to Thrive, or have severe disease unresponsive to multiple elimination diets. |
| Habel et al., 2014 [17] | DiGeorge syndrome (22q11.2 deletion syndrome) | 22q11DS UK guidelines group | Children may experience gastroesophageal reflux and dysphagia which can lead to aspiration and pneumonia. Gastrostomy feeding may be indicated in these cases. |
| Koleltzko et al., 2012 [5] | CMPA | ESPGHAN GI committee practical guidelines | AAFs are recommended for infants who react to extensively hydrolyze infant formulas. Children with severe enteropathy or multiple food allergies should receive AAFs especially those with severe anaphylactic reactions or severe enteropathy. |
| Marchand et al., 2009 [24] | Neurologic impairment | Canadian pediatric society position statement, nutrition in neurologically impaired children | After 12 months of age, most children will tolerate a polymeric formula, but some may require a semi-elemental or AAF. |
| CMPA cow’s milk protein allergy; AAF amino acid-based formula; EoE Eosinophilic esophagitis; NASPGHAN North American society for pediatric gastroenterology, Hepatology and Nutrition; ESPGHAN European society for pediatric gastroenterology, hepatology and nutrition. | |||
Table 8: Consensus statements, professional guidelines and review articles supporting the use of amino acid-based formulations. CMPA cow’s milk protein allergy; AAF amino acid-based formula; EoE Eosinophilic esophagitis; NASPGHAN North American Society for pediatric gastroenterology, Hepatology and nutrition; ESPGHAN European society for pediatric gastroenterology, Hepatology and nutrition.
Strengths and Limitations of the Study
Professional experts at our children’s rehabilitation hospital provide long-term treatment and follow-up for children with a wide range of medically complex conditions. Many of these children need enteral tube-feeding because they are unable to consume nutrition orally, which can result in nutritional deficiencies and growth failure. As such, we aimed to review electronic medical records to identify children who were switched to an AA-MCT feeding. We were also able to follow many study subjects up to 12-months post-switch, an interval sufficiently long to observe nutrition-related growth patterns.
Despite all eligible patients at this single center being included in the review, our study population sample was small (n=23) and heterogeneous. It included boys and girls aged 1 to 18 years, and these children had a wide range of medical conditions. Further, the study was a retrospective study design, no formal sample size calculation was computed, and we were only able to show associations between feeding formula and outcomes rather than cause-and-effect relationships. In addition, one subject had a prescription for oral intake at 12 months prior to switch to AA-MCT, however, the contribution of these calories and protein intake were unavailable in medical records. Gaps in the records presented some difficulty in obtaining consistent high-quality data. The learning’s from this study can be used to generate hypotheses and to drive future trials with AA-MCT formulas.
Conclusions
Feeding-formula intolerance has long been attributed to allergies, especially cow’s milk protein allergy in infancy. More recently, such feeding-formula intolerance has been associated with genetic, metabolic and neurologic conditions in which gastrointestinal dysfunction are prominent, as is common among children with medically complex conditions. Gastrointestinal dysfunction with intolerance of formula feedings is also now recognized to extend far beyond infancy.
Our study findings showed that children aged 1 to 18 years with a variety of clinical diagnoses and indications could benefit from a switch to feeding a nutritionally complete, tube-fed formula containing readily absorbable amino acids and medium-chain triglycerides. Our study population exhibited good feeding tolerance and growth, as evidenced by meeting nutritional and growth goals up to 1-year post-switch. We observed age-appropriate growth for children with a wide range of immunologic, genetic, metabolic, neurologic, and developmental conditions associated with malabsorption and some children observed a reduction in GI medications after switching to AA-MCT. Such findings indicate that specialized nutrition for medically complex children can contribute to optimal care. Expected improvements in growth and development could markedly enhance quality of life for these medically complex children.
Conflict of Interest
Pamela Cekola, Aimee Henrikson and Krysmaru Araujo Torres are employees of Nestlé Health Science. Amy Hulsey, an employee of Children's Center Rehabilitation Hospital, Heidi Reichert and Dr. Sarah Cohen, employees of EpidStrategies, a Division of ToxStrategies, Inc., all received payments from Nestlé Health Science for the work of this trial. All authors are responsible for the reported research in this manuscript and have participated in the study’s concept and design; analysis and interpretation of data; drafting or revising of the manuscript and have approved the manuscript as submitted. Some authors had primary responsibility for specific aspects of the study: conceptualization by AH, PC, AH, HR, SSC and KAT; methodology by AH, PC, AH, HR, SSC and KAT; data curation by AH, PC, HR, SSC; and data analyses by AH, PC, AH, HR, SSC and KAT.
Funding Source
Nestlé HealthCare Nutrition sponsored research.
Acknowledgement
The authors extend thanks to Kathryn Hennessy, MS, RN, FASPEN, and Cecilia Hofmann, PhD (C Hofmann & Associates, Western Springs, IL, USA) for assistance with writing and editing this manuscript.
References
- Berry JG, Hall M, Neff J, Goodman D, Cohen E, et al. (2014) Children with medical complexity and medicaid: Spending and cost savings. Health Aff (Millwood) 33: 2199-2206.
[Crossref], [Google Scholar], [Indexed]
- Vandenplas Y, Brough HA, Fiocchi A, Miqdady M, Munasir Z, et al. (2021) Current guidelines and future strategies for the management of cow's milk allergy. J Asthma Allergy 14: 1243-1256.
[Crossref], [Google Scholar], [Indexed]
- Nocerino R, Di-Scala C, Coppola S, Giglio V, Carucci L, et al. (2021) Tolerability of a new amino acid-based formula for children with IgE-mediated cow's milk allergy. Ital J Pediatr 47: 151.
[Crossref], [Google Scholar], [Indexed]
- Meyer R, Groetch M, Venter C (2018) When should infants with cow's milk protein allergy use an amino acid formula? a practical guide. J Allergy Clin Immunol Pract 6: 383-399.
[Crossref], [Google Scholar], [Indexed]
- Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, et al. (2012) Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr 55: 221-229.
[Crossref], [Google Scholar], [Indexed]
- Hill DJ, Murch SH, Rafferty K, Wallis P, Green CJ (2007) The efficacy of amino acid-based formulas in relieving the symptoms of cow's milk allergy: A systematic review. Clin Exp Allergy 37: 808-822.
[Crossref], [Google Scholar], [Indexed]
- Atwal K, Hubbard GP, Venter C, Stratton RJ (2019) The use of amino acid-based nutritional feeds is effective in the dietary management of pediatric eosinophilic oesophagitis. Immun Inflamm Dis 7: 292-303.
[Crossref], [Google Scholar], [Indexed]
- Gomez-Aldana A, Jaramillo-Santos M, Delgado A, Jaramillo C, Luquez-Mindiola A (2019) Eosinophilic esophagitis: Current concepts in diagnosis and treatment. World J Gastroenterol 25: 4598-4613.
[Crossref], [Google Scholar], [Indexed]
- Groetch M, Venter C, Skypala I, Vlieg-Boerstra B, Grimshaw K, et al. (2017) Dietary therapy and nutrition management of eosinophilic esophagitis: A work group report of the American academy of allergy, asthma and immunology. J Allergy Clin Immunol Pract 5: 312-329.
[Crossref], [Google Scholar], [Indexed]
- Papadopoulou A, Koletzko S, Heuschkel R, Dias JA, Allen KJ, et al. (2014) Management guidelines of eosinophilic esophagitis in childhood. J Pediatr Gastroenterol Nutr 58: 107-118.
[Crossref], [Google Scholar], [Indexed]
- Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, et al. (2013) American college of G. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 108: 679-692.
[Crossref], [Google Scholar], [Indexed]
- Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, et al. (2011) Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol 128: 3-26.
[Crossref], [Google Scholar], [Indexed]
- Nowak-Wegrzyn A, Chehade M, Groetch ME, Spergel JM, Wood RA, et al. (2017) International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: Executive summary-workgroup report of the adverse reactions to foods committee, American academy of allergy, asthma & immunology. J Allergy Clin Immunol 139: 1111-1114.
[Crossref], [Google Scholar], [Indexed]
- American Academy of Pediatrics (2000) Committee on nutrition. Hypoallergenic infant formulas. Pediatrics 106: 346-349.
[Crossref], [Google Scholar], [Indexed]
- Quitadamo P, Thapar N, Staiano A, Borrelli O (2016) Gastrointestinal and nutritional problems in neurologically impaired children. Eur J Paediatr Neurol 20: 810-815.
[Crossref], [Google Scholar], [Indexed]
- Penagini F, Mameli C, Fabiano V, Brunetti D, Dilillo D, Zuccotti GV (2015) Dietary intakes and nutritional issues in neurologically Impaired children. Nutrients 7: 9400-9415.
[Crossref], [Google Scholar], [Indexed]
- Habel A, Herriot R, Kumararatne D, Allgrove J, Baker K, et al. (2014) Towards a safety net for management of 22q11.2 deletion syndrome: Guidelines for our times. Eur J Pediatr 173: 757-765.
[Crossref], [Google Scholar], [Indexed]
- Vajro P, Maddaluno S, Veropalumbo C (2013) Persistent hyper transaminasemia in asymptomatic children: A stepwise approach. World J Gastroenterol 19: 2740-2751.
[Crossref], [Google Scholar], [Indexed]
- Diskin C, Malik K, Gill PJ, Rashid N, Chan CY, et al. (2022) Research priorities for children with neurological impairment and medical complexity in high-income countries. Dev Med Child Neurol 64: 200-208.
[Crossref], [Google Scholar], [Indexed]
- Kleinert JO (2017) Pediatric feeding disorders and severe developmental disabilities. Semin Speech Lang 38: 116-125.
[Crossref], [Google Scholar], [Indexed]
- Minor G, Yamamoto S, Cekola P, Cohen S, Huhmann M, et al. (2020) Meeting nutritional needs of the enterally-fed child with neurological impairment. J Clin Nutr Diet 6: 1-9.
[Crossref], [Google Scholar]
- Romano C, Van-Wynckel M, Hulst J, Broekaert I, Bronsky J, et al. (2017) European society for paediatric gastroenterology, hepatology and nutrition guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with neurological impairment. J Pediatr Gastroenterol Nutr 65: 242-264.
[Crossref], [Google Scholar], [Indexed]
- Romano C, Dipasquale V, Gottrand F, Sullivan PB (2018) Gastrointestinal and nutritional issues in children with neurological disability. Dev Med Child Neurol 60: 892-896.
[Crossref], [Google Scholar], [Indexed]
- Marchand V, Canadian Paediatric Society Nutrition and Gastroenterology Committee (2009) Nutrition in neurologically impaired children. Paediatr Child Health 14: 395-401.
- Miele E, Staiano A, Tozzi A, Auricchio R, Paparo F, et al. (2002) Clinical response to amino acid-based formula in neurologically impaired children with refractory esophagitis. J Pediatr Gastroenterol Nutr 35: 314-319.
[Crossref], [Google Scholar], [Indexed]
- Brooks J, Day S, Shavelle R, Strauss D (2011) Low weight, morbidity and mortality in children with cerebral palsy: new clinical growth charts. Pediatrics 128: e299-307.
[Crossref], [Google Scholar], [Indexed]
- Hanna SE, Bartlett DJ, Rivard LM, Russell DJ (2008) Reference curves for the gross motor function measure: Percentiles for clinical description and tracking over time among children with cerebral palsy. Phys Ther 88: 596-607.
[Crossref], [Google Scholar], [Indexed]
- https://canchild.ca/en/resources/42-gross-motor-function-classification-system-expanded-revised-gmfcs-e-r.
- Cohen E, Kuo DZ, Agrawal R, Berry JG, Bhagat SK, (2011) Children with medical complexity: An emerging population for clinical and research initiatives. Pediatrics 127: 529-538.
[Crossref], [Google Scholar], [Indexed]
- Gargano D, Appanna R, Santonicola A, De-Bartolomeis F, Stellato C, et al. (2021) Food allergy and intolerance: A narrative review on nutritional concerns. Nutrients 13.
[Crossref], [Google Scholar], [Indexed]
- Calvani M, Anania C, Cuomo B, D'Auria E, Decimo F, et al. (2021) Non-IgE- or mixed IgE/Non-IgE-mediated gastrointestinal food allergies in the first years of life: Old and new tools for diagnosis. Nutrients 13.
[Crossref], [Google Scholar], [Indexed]
- Nowak-Wegrzyn A, Jarocka-Cyrta E, Moschione CA (2017) Food protein-induced enterocolitis syndrome. J Investig Allergol Clin Immunol 27: 1-18.
[Crossref], [Google Scholar], [Indexed]
- Sampson HA (1999) Food allergy. Part 2: Diagnosis and management. J Allergy Clin Immunol 103: 981-989.
[Crossref], [Google Scholar], [Indexed]
- Host A (1994) Cow's milk protein allergy and intolerance in infancy. Some clinical, epidemiological and immunological aspects. Pediatr Allergy Immunol 5: 1-36.
[Crossref], [Google Scholar], [Indexed]
- Mayer O, Kerner JA (2017) Management of short bowel syndrome in postoperative very low birth weight infants. Semin Fetal Neonatal Med 22: 49-56.
[Crossref], [Google Scholar], [Indexed]
- Warren CM, Jiang J, Gupta RS (2020) Epidemiology and burden of food allergy. Curr Allergy Asthma Rep 20: 6.
[Crossref], [Google Scholar], [Indexed]
- Adams CB, Johnston WH, Deulofeut H, Leader J, Rhodes R, et al. (2021) Growth and tolerance of healthy, term infants fed lower protein extensively hydrolyzed or amino acid-based formula: double-blind, randomized, controlled trial. BMC Pediatr 21: 323.
[Crossref], [Google Scholar], [Indexed]
- Vandenplas Y, Dupont C, Eigenmann P, Heine RG, Host A, et al. (2021) Growth in infants with cow's milk protein allergy fed an amino acid-based formula. Pediatr Gastroenterol Hepatol Nutr 24: 392-402.
[Crossref], [Google Scholar], [Indexed]
- Cekola P, Henrikson A, Reichert H, Cohen S, Huhmann M, et al. (2021) Safety and usage of an amino acid-based formula for infants: Results from a post market surveillance study. J Clin Nutr Diet 7: 8309.
[Crossref], [Google Scholar]
- Fierro V, Valluzzi RL, Banzato C, Plaza MA, Bosque M, et al. (2020) A well-tolerated new amino acid-based formula for cow's milk allergy. Immun Inflamm Dis 8: 140-149.
[Crossref], [Google Scholar], [Indexed]
- Canani RB, Nocerino R, Frediani T, Lucarelli S, Di-Scala C, et al. (2017) Amino acid-based formula in cow's milk allergy: Long-term effects on body growth and protein metabolism. J Pediatr Gastroenterol Nutr 64: 632-638.
[Crossref], [Google Scholar], [Indexed]
- Vanderhoof J, Moore N, De-Boissieu D (2016) Evaluation of an amino acid-based formula in infants not responding to extensively hydrolyzed protein formula. J Pediatr Gastroenterol Nutr 63: 531-533.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences