Formula Switch Leads to Enteral Feeding Tolerance Improvements in Adults: A Case Series
Bethany Hopkins1*, Maureen Huhmann2, Seletha Periman3, Sarah S. Cohen4 and Jean Chouinard4
Bethany Hopkins1*, Maureen Huhmann2, Seletha Periman3, Sarah S. Cohen4 and Jean Chouinard5
1Department of Nutrition and Dietetics, Sheppard Ave West North York, NHS Foundation Trust, Ontario, Canada
2Department of Nutrition and Dietetics, Nestle HealthCare Nutrition, US Highway, Building JR2 Bridgewater NHS Foundation Trust, Bridgewater, United States
3Department of Nutrition and Dietetics, EpidStrategies, a division of ToxStrategies, Kildaire Farm, United States
4Nestlé Health Science, Bruyere Research Institute, St Vincent Hospital60 Cambridge St. N, Ottawa, ON, Canada
5Bruyere Research Institute, St VincentHospital, 60 Cambridge St. N, Ottawa, ON,Canada
- *Corresponding Author:
- Bethany Hopkins
Department of Nutrition and Dietetics
Sheppard Ave West North York
NHS Foundation Trust
Ontario
Canada
E-mail: bethany.hopkins@ca.nestle. com
Received Date: June 01, 2021 Accepted Date: June 18, 2021; Published Date: June 25, 2021
Citation: Hopkins B, Huhmann M, Periman S, Cohen SS, Chouinard J (2021) Formula Switch Leads to Enteral Feeding Tolerance Improvements in Adults: A Case Series. J Clin Nutr Diet Vol.7 No.7:3.
Abstract
Enteral Nutrition (EN) is a life-sustaining treatment for individuals across care settings. EN Intolerance (ENI) is a common complication associated with EN. A variety of strategies are utilized to manage ENI, including the switch from polymeric to Peptide-Based Diets (PBD). Despite the necessity of EN and the prevalence of reported gastrointestinal complications, there remains a paucity of published data regarding ENI and potential benefits of PBD as a management strategy. This case series reports our experience with ENI in adults living in a complex continuing care facility. A total of 10 eternally-fed patients (4 women and 6 men) with ENI, average age of 64 years [± 10], were switched from a polymeric formula to a 100% whey PBD. Data on overall feeding tolerance was available for 8/10 patients. In all eight, feeding tolerance was reported as improved after switch. Of the ENI symptoms reported at baseline, improvements were observed in excessive gas or abdominal distention (2/4 cases); loose stool (4/6 cases); constipation (1/2 cases); elevated gastric residuals (2/2 cases); and “other” signs of intolerance (4/6 cases). Vomiting was unchanged (1/2 cases) or worsened (1/2 cases). Of the identified ENI-related medications used prior to formula switch, complete data was available for 8/10 patients. Of these, 4/8 patients had a reduction or discontinuation in medications after switch. Switching to 100% whey PBD was associated with improved feeding tolerance in this small cohort of patients experiencing ENI. These results are promising; however, additional trials are needed to better understand the true benefits.
Keywords
Enteral nutrition; Enteral formulas; Enteral nutrition Intolerance; Peptide-based diet
Introduction
Enteral Nutrition (EN) is an important, life-sustaining treatment for individuals with functional Gastrointestinal (GI) tracts who are unable to consume adequate oral intakes [1-4]. EN is used in patients with diverse clinical and medical conditions across care settings from hospital to homecare. Despite the increasing prevalence of EN, there are inherent risks of complications associated with its use, including GI complications [3,4]. Enteral Nutrition Intolerance (ENI), a frequently cited GI complication, may interfere with successful delivery of EN and have a considerable impact on patients, caregivers and healthcare utilization [4,5]. ENI is often described by the presence of GI symptoms including nausea, vomiting, reflux, abdominal distension, diarrhoea and constipation [5-8]. Incidence of ENI has been reported to range from 25%-75% and is associated with reduced nutrition delivery, decreased quality of life and adverse clinical and health economic outcomes [8-14]. In a recent survey of 240 Registered Dieticians (RDs), ENI symptoms were reported to affect between 35-66% of patients across settings (acute care, home care, long term care) with reduction in volume of EN delivered reported as a common management approach [15]. Other authors have reported similar approaches, with reductions in feeding rates and stopping feeding infusions having implications for inadequate nutrition delivery [5-16].
Case Presentation
There is no standard approach to the management of ENI, in their review of GI complications during EN, Btaiche and colleagues suggested that simple measures can be taken to facilitate successful EN delivery [12]. One such measure may be a change in formula provided. Outside the ICU setting, Canadian RDs reported using this as a method of managing ENI approximately 20% of the time [15]. More recently, Mundi et al. described experiences from a large adult home enteral nutrition program in the US where peptide-based diets (PBD) were used in patients who were unable to tolerate standard polymeric formulas [4]. In 95 patients from their home enteral nutrition program, use of PBD resulted in significant improvements in ENI symptoms and reductions in healthcare utilization (patient-initiated phone calls, visits to the emergency department and scheduled care-provider visits [4]. In the podiatric nutrition community, PBD are long known to play a role in feeding tolerance, owing in part to the unique digestion kinetics of hydrolyzed whey protein, which facilitates gastric emptying in children with a range of upper GI motility disorders [17-19]. Minor et al. reported this in a small group of developmentally delayed children wherein 92% had improvement in tolerance parameters when switched from an intact protein formula to 100% whey PBD [19].
To help assess the impact of changing EN formula as a strategy to manage intolerance, we reviewed the cases of adult complex care patients switched to a PBD due to ENI. We hypothesized that switching to PBD would improve tolerance and result in improvements in reported ENI symptoms.
Methods/Case Presentation
This case series describes the experience with managing ENI in 10 adult enterally fed patients by switching to a 100% whey PBD. Patients were identified using a retrospective review of real-world practice data in enterally-fed adults experiencing ENI. The facility at which this study was conducted is described as a complex, continuing care facility for medically complex adult patients who require regular on-site physician/ nursing care and assessment; and active care management by a multidisciplinary team of specialized staff which includes RDs. The inpatient program at this facility cares for patients on long-term EN that receive a wide variety of commercially available enteral formulas including both polymeric formulas and PBD. The staff RD individualizes the EN formula, schedule, and prescribed dose based on the patients’ clinical condition and needs.
Primary inclusion criteria were 1) prescribed EN to provide ≥ 90% of estimated daily calorie and protein needs; 2) switched from a polymeric formula to a 100% whey PBD formula due to reported ENI; 3) have complete baseline data on ENI while using a polymeric formula; and 4) received PBD for at least 2 weeks post-formula switch. Patients were excluded from the case series if they had recent abdominal surgery and/or infections at time of formula switch.
Data were collected from medical health records over the last 10 years. Demographic data collected at baseline included age, gender, enteral feeding route, height/weight/body mass index (BMI; kg/m2) prior to and 30 days after formula switch, admission diagnoses, and all diagnoses at time of switch. The rationale for formula switch and GI tolerance pre- and post-switch was recorded, in addition to use of any medications to manage ENI. Tolerance parameters assessed included volume of formula infused versus goal, nausea and vomiting, residuals, gagging/ retching, abdominal gas/distention, and stool assessments. Overall assessment of tolerance to PBD by healthcare providers were categorized as “Improved”, “No change”, or “Worsened” based on information documented in progress notes and clinician assessments/consult reports. Basic descriptive statistics were used with data presented as mean ± standard deviation for continuous data or counts and percentages for categorical data.
This study was approved by the Bruyère Continuing Care Research Ethics Board (Ottawa, ON, CA). Informed consent was required and obtained for all patients enrolled who were in active care at the time of the study.
Results
A total of 10 patients (4 women, 6 men) with an average age of 64 years [± 10], were switched from a polymeric formula to a 100% whey PBD to help manage their ENI (Table 1). The majority were fed via gastrostomy feeding tubes (80%). Half of the patients had a neurological admission diagnosis [n=5]. The numbers of reported concomitant diagnoses at time of EN feeding switch were recorded, with most patients having between 5-10 secondary diagnoses ranging from a variety of neurological, cardiovascular, renal, metabolic and mental health conditions (data not shown). The most common indications for formula switch to a PBD was diarrhoea/loose stool (42%) and upper GI symptoms (42%) (Table 1). Patients had been on a polymeric formula for an average of 25 weeks [range 2–68] before the switch.
| Variable | N=10 |
|---|---|
| Agea | 63.8 ± 10 |
| Gender (women/men) | 4/6 |
| Feeding routeb | 8 G-Tube; 1 J-tube |
| Admitting diagnoses | |
| Hemorrhagic stroke | 2 (20%) |
| Neurodegenerative disease | 2 (20%) |
| Cancer (1, head and neck; 1, ovarian) | 2 (20%) |
| Dementia | 1 (10%) |
| Zollinger-Ellison Syndrome | 1 (10%) |
| Esophageal perforation | 1 (10%) |
| Respiratory Illness | 1 (10%) |
| Duration of polymeric EN formula use prior to switch to PBD | 25 weeks [2 – 68]c |
| Primary reason for EN formula switch to PBDd | N (% out 12)d |
| Diarrhea/loose stool | 5 (42%) |
| Delayed gastric emptying/ Abdominal distension | 3 (25%) |
| Nausea/Vomiting/Reflux | 2 (16.5%) |
| Other | 2 (16.5%) |
Note: (a) Values are mean ± standard deviation; (b) Documented route missing for 1 patient; (c) values are mean [range] (d) More than one reason may have been provided for a patient, so N sum is greater than the total # of patients EN, enteral nutrition; G-tube, gastrostomy feeding tube; J-tube, jejunostomy feeding tube; PBD, peptide-based diet.
Table 1: Patient demographics.
Prior to formula switch, patients’ initial mean BMI was 23.6 ± 4.7 kg/m2 and they were prescribed an average of 1517 kcal (± 452) and 75 g protein (± 15) per day (Table 2). Half of the patients were on an intermittent EN schedule with 3–4 feedings/day (237–375 mL/per feeding). The remaining five patients were fed at lower rates (20-65 mL/hr) via pump over 7–24 hours/day. The caloric density of the feedings for each patient ranged from 1 kcal/mL (2/10), 1.5 kcal/mL (6/10); 2 kcal/mL (1/10); to a combination of feedings of different caloric densities (1/10). Eight patients (80%) were on fibre containing polymeric EN formulas prior to switch. After switch, nine patients transitioned to a 1.5 kcal/ mL 100% whey PBD (Peptamen®1.5, Nestlé Health Science) and one patient transitioned to a 1.2 kcal/mL 100% whey PBD (Peptamen®AF 1.2, Nestlé Health Science). Prescribed feeding schedules were similar post switch.
| Variable | Pre-Switch to PBD | Post-Switch to PBD |
|---|---|---|
| BMI (kg/m2) | 23.6 ± 4.7 | 24.3 (± 8.1)a |
| Weight (kg) | 66.6 ± 9.4 | 68.7 (± 17.4)a |
| Total calories per day (kcal)b | 1517 (± 452) | 1525 (± 323) |
| Total protein per day (grams)b | 75 (± 15) | 78 (± 12) |
Abbreviations: BMI: Body Mass Index; PBD: Peptide-Based Diet
Note: (a) 30 days post-switch to PBD (b) Calorie and protein intake based on documented prescribed feeding regimes. Details with respect to prescribed vs. actual intake were not clear in most patients’ medical records.
Table 2: Pre-switch to PBD and Post-PBD values from 10 patients.
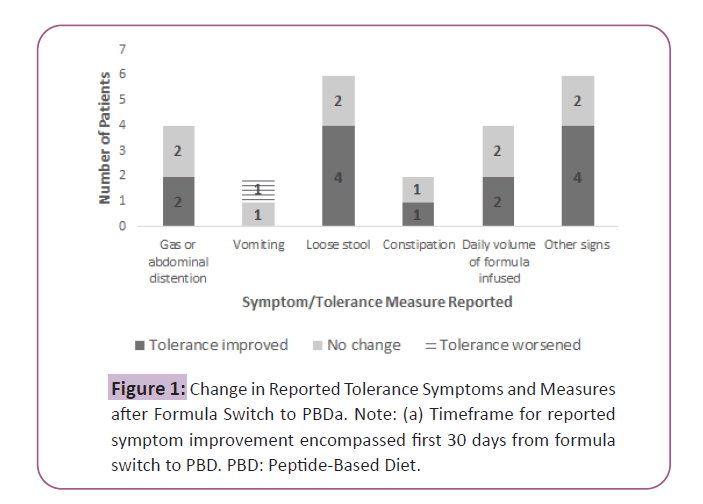
After switch to the PBD, patients maintained their weight with an average BMI at 30 days of 24.3 kg/m2 (± 8.1) and energy and protein prescriptions were similar (Table 2). With respect to the specific GI symptoms and intolerance measures reported, improvements were observed in: excessive gas or abdominal distention (2/4 cases); loose stool (4/6 cases); constipation (1/2 cases); daily volume of formula infused (2/4 cases) and other signs of intolerance (4/6 cases; reported improvements in weight loss or inadequate intake and/or abdominal pain) (Figure 1). Overall feeding tolerance, based on the RDs documented clinical assessment, was reported in 8/10 patients.
ENI was reported as improved after switch in all eight. Complete data on use of medications related to managing tolerance was available in 8/10 patients. Half of the patients (4/8) had a reduction or discontinuation in feeding intolerance-related medications after the switch (motility agents, stool softeners, laxatives, anti-emetics).
Discussion
This case series describes the experience in a complex continuing care facility with 10 adult patients with multiple co-morbidities who were switched to a PBD because of ENI which presented with a variety of upper and lower GI symptoms including diarrhoea/ loose stools, delayed gastric emptying/abdominal distension and nausea/vomiting/reflux. This group of patients maintained their weight and BMI (kg/m2) when switched to a PBD. More importantly, in all eight patients with complete data available on the RDs assessment of intolerance, their EN tolerance was reported to improve. In addition, there were reductions observed in use of medications to manage ENI. These findings are similar to those reported by Minor et al., who reported switching to 100% whey PBD improved symptoms of feeding intolerance and resulted in medication changes in a group of 13 developmentally delayed children [19]. Likewise, Mundi found significant improvements in nausea, vomiting, diarrhoea, abdominal pain and distention in home EN patients who were switched to PBD [4].
ENI, which frequently presents as upper and lower GI symptoms, is a commonly reported complication associated with EN [4- 15]. In this case series, the symptoms which prompted a switch to PBD were either a mix of upper GI symptoms (delayed gastric emptying, abdominal distention, nausea, vomiting and reflux) or diarrhoea. This is consistent with existing literature. Despite the wide variability in definition and lack of a consistent approach to quantifying stool output, diarrhea is the most frequently cited ENI GI symptom among eternally fed patients, across care settings [8-15]. Upper GI symptoms may be equally troubling and are commonly reported in the ENI literature, with delayed gastric emptying reported as the primary contributor to nausea and vomiting in EN patients and EN interruptions are most often attributed to abdominal distention [8]. In a recent survey of Canadian RDs about their patients’ experience with ENI, diarrhoea was reported in almost one-third, with upper GI symptoms reported in over half of patients [15].
Management of ENI GI symptoms may involve a number of strategies including use of medications, reducing the volume of formula delivered, switching EN formulas and feeding schedules, discontinuation of EN and use of Parenteral Nutrition (PN) [5- 16]. Concerns have been raised regarding delivery of adequate nutrition and hydration with cessation and frequent stopping of EN infusions and approach to deal with ENI [5-20]. As introduced earlier, strategies to help manage ENI may involve simple steps, such as switching EN formula. In a recent review by Mundi et al. of their home EN database, use of PBD was a viable option in the management of enterally fed patients with ENI [4]. A study by Hopkins et al. reported that RDs EN practices for managing ENI found formula switch was reported as a management strategy in 20% of patients [15]. Based on the results of this case series, combined with the work reported by Mundi et al. [4] and Minor et al. [19], we believe there may be a role for 100% whey PBD as a simple strategy to consider in managing ENI.
PBD have been used in patients who have difficulty digesting and absorbing standard polymeric diets or in those who have challenges achieving adequate nutrition [21]. Free amino acids and peptides are then transported into mucosal cells [22]. We know from literature on peptide transport that amino acids from protein are more readily absorbed in peptide form and that changes in peptide transport expression may occur with alterations in nutrition status, disease and illness, which may have implications for the management of some individuals on EN [23,24]. In addition, the source of protein may also be a factor to consider in the management of ENI. Whey protein is considered to be a ‘fast protein’, in part related to its ability to remain soluble in an acidic pH, which has implications for gastric emptying in addition to postprandial protein accretion [25,26]. In Abrahao’s review of gut dysfunction in illness, whey protein is suggested to play a role in better tolerance of enterally fed patients [27].
The second aspect of PBD, which may play a role in tolerance, is related to the lipid content. PBDs typically contain a higher percentage of Medium-Chain Triglycerides (MCT) as compared to standard polymeric formulas. MCT are triglycerides containing [6-12]. carbon chain fatty acids which were first synthesized in the 1950’s and sometimes referred to as one of the first medical foods [28,29]. Unlike LCT, MCT are more water-soluble and they do not require pancreatic lipase, emulsification from bile salts, micelle formation or re-packaging into chylomicrons for transportation via the lymphatic system [28-30].Because of their unique chemical and physical properties, MCT allow for faster and more efficient hydrolysis and absorption and may play an important role as an alternative lipid source for patients who are ill or those with impaired or dysfunctional GI tracts [4-31].
In the face of these theoretical benefits of PBD, we speculate use of these EN formulas may be overlooked in the clinicians’ toolbox when looking for solutions to manage ENI. One of the reasons for the hesitation to use PBD may be related to their higher cost as compared to polymeric formulas, as noted by Mundi et al. in their home EN practice [4]. When considering the financial cost of EN and ENI, other considerations should be taken into account including the cost to the patient’s quality of life, the nutrition deficits (which may ensue due to inadequate nutrition over days and weeks of intolerance), distress to caregivers and the cost to the healthcare system. ENI may have a deleterious affect across all of these domains [4-15]. The experience of these patients may be similar to other individuals on long-term tube feeding where improvements in GI symptoms may have implications for quality of life and nutrition delivery in complex adult patients requiring longstanding EN. In home EN patients, use of PBD not only improved symptoms of ENI for patients, but also resulted in savings related to health care utilization with fewer phone calls to providers, visits to emergency departments and provider visits [4].
We acknowledge that our case series has limitations. Given our small sample size, our observations may not be representative of the overall adult EN population which limits the generalizability of this case series. This makes data extraction laborious and there may be incomplete or inaccurate information in the medical records, which limits the quality of data available for analysis and interpretation. Despite these limitations, real-world evidence provides an opportunity to gain insights into clinical practice and the benefits of interventions to patients encountered by clinicians in facilities.
Conclusion
Our case series demonstrates that switching to 100% whey PBD was associated with improved feeding tolerance in this small group of eternally fed adults experiencing ENI. Prospective randomized control trials, additional real-world evidence and health economic data are necessary to understand the full impact of switching to PBD to manage ENI.
Conflicts of Interest
This study was funded by Nestlé Health Science Canada. B. Hopkins, M. Huhmann and S. Periman are employees and receive a salary from Nestlé Health Science. S. Cohen is employed by EpidStrategies (formerly EpidStat Institute) which received payment from Nestlé Health Science for the analysis of this research.
Funding
This study was funded by Nestlé Health Science Canada.
References
- Wong A, Banks MD, Bauer JD (2018) A survey of home enteral nutrition practices and reimbursement in the asia pacific region. Nutrients 10:214.
- Gramlich L, Hurt RT, Jin J, Mundi MS (2018) Home enteral nutrition: Towards a standard of care. Nutrients 10:1020
- Boullata JI, Carrera AL, Harvey L, Escuro AA, Hudson L et al. (2017) ASPEN safe practices for enteral nutrition therapy [Formula: see text]. JPEN J Parenter Enteral Nutr 41:15-103
- Mundi MS, Velapati S, Kuchkuntla AR, Hurt RT (2020) Reduction in healthcare utilization with transition to peptide-based diets in intolerant home enteral nutrition patients. Nutr Clin Pract 35:487-494
- Wang K, McIlroy K, Plank LD, Petrov MS, Windsor JA (2017) Prevalence, outcomes, and management of enteral tube feeding intolerance: A retrospective cohort study in a tertiary center. JPEN J Parenter Enteral Nutr 41:959-967
- Malone A, Seres D, Lorde L (2012) Complications of enteral nutrition. In: Mueller CM (ed) The A.S.P.E.N. adult nutrition support core curriculum. 2nd ed. Silver Spring, MD: A.S.P.E.N.218-233.
- Bernard AC, Magnuson B, Tsuei BJ, Swintosky M, Barnes S et al. (2004) Defining and assessing tolerance in enteral nutrition. Nutr Clin Pract 19:481-6
- Boullata J, Carney LN, Guenter P (eds) (2010) A.S.P.E.N. Enteral Nutrition Handbook. Silver Spring, MD: A.S.P.E.N.
- Gungabissoon U, Hacquoil K, Bains C, Irizarry M, Irizarry G (2015) Prevalence, risk factors, clinical consequences, and treatment of enteral feed intolerance during critical illness. JPEN J Parenter Enteral Nutr 39:441-83
- Blaser A, Starkopf J, Kirsimägi Ü, Deane AM (2014) Definition, prevalence, and outcome of feeding intolerance in intensive care: A systematic review and meta-analysis. Acta Anaesthesiol Scand 58:914-22
- Heyland DK, Dhaliwal R, Wang M, Andrew G Day (2014) The prevalence of iatrogenic underfeeding in the nutritionally 'at-risk' critically ill patient: Results of an international, multicenter, prospective study. Clin Nutr 34:659-66
- Btaiche IF, Chan LN, Pleva M, Kraft MD (2010) Critical illness, gastrointestinal complications, and medication therapy during enteral feeding in critically ill adult patients. Nutr Clin Pract 25:32-49
- Kozeniecki M, Fritzshall R (2015) Enteral nutrition for adults in the hospital setting. Nutr Clin Pract 30:634-51
- Virani FR, Peery T, Rivas O, Tomasek J, Huerta R (2019) Incidence and effects of feeding intolerance in trauma patients. JPEN J Parenter Enteral Nutr 43:742-749
- Hopkins B, Donnelly-Vanderloo M,Davis B, Madill J (2017) Prevalence and management of enteral nutrition intolerance in the non-ICU setting in canada. The Canadian Journal of Clinical Nutrition.5:1-20
- Stewart M L (2014) Interruptions in enteral nutrition delivery in critically ill patients and recommendations for clinical practice. Crit Care Nurse 34:14-21
- Fried MD, Khoshoo V, Secker DJ, Gilday DL, Ash JM et al. (1992) Decrease in gastric emptying time and episodes of regurgitation in children with spastic quadriplegia fed a whey-based formula. J Pediatr 120:569-72.
- Savage K, Kritas S, Schwarzer A, Davidson G, Omari T (2012)Whey- vs casein-based enteral formula and gastrointestinal function in children with cerebral palsy. JPEN J Parenter Enteral Nutr 36:118S-23S.
- Minor G, Ochoa JB, Storm H, Periman S (2016) Formula switch leads to enteral feeding tolerance improvements in children with developmental delays .Glob Pediatr Health
- Tappenden KA, Quatrara B, Parkhurst ML, Malone AM, Fanjiang G et al. (2013) Critical role of nutrition in improving quality of care: An interdisciplinary call to action to address adult hospital malnutrition. JPEN J Parenter Enteral Nutr 37:482-97
- Alexander DD, Bylsma LC, Elkayam L, Nguyen DL (2016) Nutritional and health benefits of semi-elemental diets: A comprehensive summary of the literature. World J Gastrointest Pharmacol Ther 6;7:306-19
- Silk DB, Grimble GK, Rees RG (1985) Protein digestion and amino acid and peptide absorption. Proc Nutr Soc 44:63-72
- Freeman HJ (2015) Clinical relevance of intestinal peptide uptake. World J Gastrointest Pharmacol Ther 6: 22–27
- Daniel H (2003)Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol 66:361-84
- Dangin M, Boirie Y, Guillet, C, Beaufrère B(2002) Influence of the protein digestion rate on protein turnover in young and elderly subjects. J Nutr 132:3228S-33S
- Boirie Y, Dangin M, Gachon P, M Vasson P, Maubois JL (1997) Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA 94:14930-5
- Abrahão V (2012) Nourishing the dysfunctional gut and whey protein. Curr Opin Clin Nutr Metab Care.15:480-4
- Bach AC, Babayan VK (1982) Medium-chain triglycerides: an update. Am J Clin Nutr 36:950-62
- Wanten GJ, Naber AH (2004) Cellular and physiological effects of medium-chain triglycerides. Mini Rev Med Chem 4:847-57
- Hise M, Brown JC. Lipids. In: Mueller CM (ed) The A.S.P.E.N. nutrition support core curriculum. 2nd ed. Silver Spring, MD: A.S.P.E.N.; 2012:63-82.
- Los-Rycharska E, Kieraszewicz Z,Czerwionka-Szaflarska M (2016) Medium chain triglycerides (MCT) formulas in paediatric and allergological practice. Prz Gastroenterol 11: 226–231
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences